Soil Amoebae Are Unexpected Hotspots of Environmental Antibiotics and Antibiotic Resistance Genes
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Antibiotic resistance poses a significant threat to human health. While most studies focus on bacteria, interactions between antibiotics and other crucial microbial groups like protists remain uncertain. This study investigates how protists interact with antibiotics and examines how these interactions impact the fate of resistance genes. It reveals that amoebae exhibit high resistance to eight high-risk environmental antibiotics, accumulating significant quantities within their cells. Wild amoeboid strains from distant locations carry substantial antibiotic resistance genes (ARGs) and metal resistance genes (MRGs), with significant heterogeneity within a single species. Amoeboid symbionts and pathogens predominantly carry these genes. Paraburkholderia symbionts have reduced genomes and fewer resistance genes compared to free-living strains, while amoeba-endogenous Stenotrophomonas maltophilia does not exhibit a significantly reduced genome size. This suggests that the amoeboid hosts serve as a temporary medium facilitating its transmission. In summary, the study unveils that soil amoebae represent unexpected hotspots for antibiotics and resistance genes. Future research should assess the effects of antibiotics on often-overlooked protists and explore their role in spreading ARGs and MRGs in ecosystems. Incorporating protists into broader antibiotic resistance research is recommended, highlighting their significance within a One Health perspective.
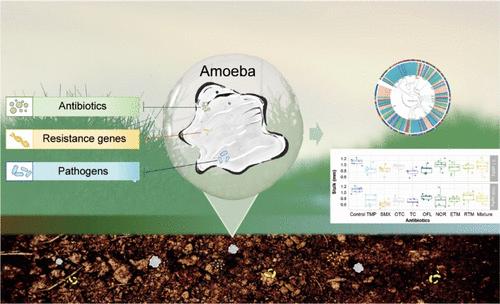
土壤变形虫是环境抗生素和抗生素耐药基因的意外热点
抗生素耐药性对人类健康构成重大威胁。虽然大多数研究都侧重于细菌,但抗生素与原生动物等其他重要微生物群之间的相互作用仍不确定。本研究调查了原生动物如何与抗生素相互作用,并探讨了这些相互作用如何影响抗药性基因的命运。研究发现,变形虫对八种高风险环境抗生素表现出很强的耐药性,并在细胞内大量积累。来自遥远地方的野生变形虫菌株携带大量抗生素抗性基因(ARGs)和金属抗性基因(MRGs),在单一物种内具有显著的异质性。变形虫共生体和病原体主要携带这些基因。与自由生活的菌株相比,副泡霍尔德氏菌共生体的基因组较小,抗性基因较少,而阿米巴内源嗜麦芽霉单胞菌的基因组大小并没有明显减少。这表明,变形虫宿主是促进其传播的临时媒介。总之,这项研究揭示了土壤变形虫是抗生素和抗性基因意想不到的热点。未来的研究应评估抗生素对经常被忽视的原生动物的影响,并探索它们在生态系统中传播 ARGs 和 MRGs 的作用。建议将原生动物纳入更广泛的抗生素耐药性研究中,从 "一体健康 "的角度强调它们的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


