Kr Adsorption in Porous Carbons: Temperature-Dependent Experimental and Computational Studies†
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
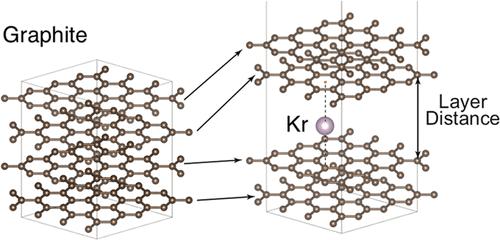
The temperature dependence of the adsorption energy of krypton adsorption on activated carbon materials was studied by experiment and simulation. Adsorption isotherms were measured at temperatures from 250 to 330 K and analyzed with Henry’s law. The adsorption energy determined from these measurements was found to weaken by more than 10% in this range. Slit pore widths for simulations in this work were modeled by the removal of integral numbers of planes in graphite. Vibrational dynamics of the krypton adsorbate and the carbon atom adsorbent were calculated with the stochastic temperature-dependent effective potential (sTDEP) method, using energetics from density functional theory (DFT) with the many-body dispersion energy method (MBD). Thermal displacements of carbon atoms had a negligible effect on the adsorption energy. The width of the slit pore had the greatest effect on the surface dynamics and the energies of the adsorbate atoms at different temperatures. Assuming a distribution of pore widths, the Boltzmann distribution of site occupancies causes a large weakening of the thermally averaged adsorption energy at higher temperatures.
多孔碳中的 Kr 吸附:与温度有关的实验和计算研究†
通过实验和模拟研究了氪在活性炭材料上的吸附能与温度的关系。在 250 至 330 K 的温度下测量了吸附等温线,并用亨利定律进行了分析。根据这些测量结果确定的吸附能在此范围内减弱了 10%以上。这项工作中模拟的狭缝孔隙宽度是通过去除石墨中的整数平面来建模的。氪吸附剂和碳原子吸附剂的振动动力学是通过随机温度相关有效势(sTDEP)方法,利用密度泛函理论(DFT)中的多体色散能方法(MBD)计算得出的。碳原子的热位移对吸附能的影响可以忽略不计。狭缝孔隙的宽度对不同温度下的表面动力学和吸附原子的能量影响最大。假定孔隙宽度分布,位点占有率的玻尔兹曼分布导致热平均吸附能在较高温度下大幅减弱。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


