Photoexcitation Dynamics of V-PYRRO/NO Investigated Using Femtosecond Time-Resolved Infrared Spectroscopy
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Diazeniumdiolates spontaneously release nitric oxide (NO) in aqueous solutions. Therefore, protected diazeniumdiolates have been developed for the controlled administration of NO to specific targets. Diazeniumdiolates with photoprotecting groups are useful for spatiotemporal NO delivery. To develop photoactivated NO donors, understanding the photodissociation dynamics of photoprotected diazeniumdiolates is essential. The dynamics of photoexcited V-PYRRO/NO (a well-studied liver-selective NO prodrug) was investigated to understand the photodissociation mechanism of protected diazeniumdiolates at the molecular level. Upon excitation at 305 nm, the N═N bond of V-PYRRO/NO was cleaved within 0.3 ps, producing N-nitrosopyrrolidine and CH2═CHON. CH2═CHON, the first oxynitrene directly observed in the solution in real-time, was formed in the singlet state and rearranged into CH2═CHNO with a time constant of 16 ± 5 ns. The calculated potential energy surfaces of the excited states confirmed the unusual breakage of the N═N bond. The findings can be utilized to develop more effective photoactivated diazeniumdiolates.
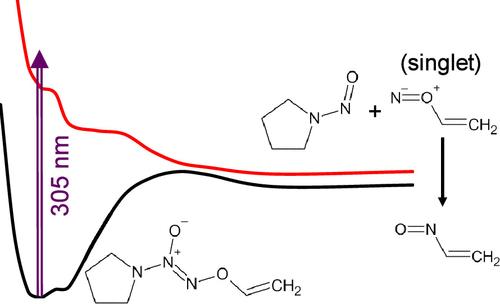
利用飞秒时间分辨红外光谱研究 V-PYRRO/NO 的光激发动力学
重氮二醇酸盐会在水溶液中自发释放一氧化氮(NO)。因此,人们开发了受保护的重氮二元醇盐,用于向特定靶点控制性地施放一氧化氮。带有光保护基团的重氮二元醇盐可用于时空一氧化氮的给药。要开发光活化 NO 给体,了解光保护重氮二醇的光解离动力学至关重要。我们研究了光激发 V-PYRRO/NO(一种经过充分研究的肝脏选择性 NO 原药)的动力学,以了解分子水平上受保护的重氮二醇的光解离机制。在 305 纳米波长的激发下,V-PYRRO/NO 的 N═N 键在 0.3 ps 内裂解,生成 N-亚硝基吡咯烷和 CH2═CHON。CH2═CHON 是第一个在溶液中实时直接观察到的炔芘,它以单线态形成,并以 16 ± 5 ns 的时间常数重新排列为 CH2═CHNO。计算得出的激发态势能面证实了 N═N 键的异常断裂。这些发现可用于开发更有效的光活化重氮二元醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


