From Molecular to Polymeric Donors: Prolonged Charge Separation in Modular Photoredox-Active Ru(II) Polypyridyl-Type Triads
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
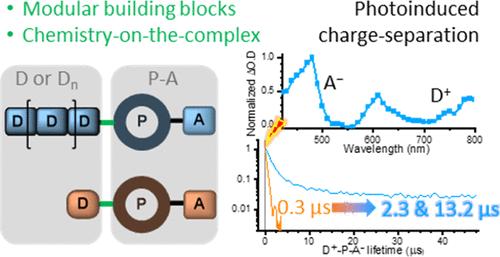
In this contribution, the divergent modular synthesis of photoredox-active dyads, triads and a tetrad descending from one ligand precursor is presented by combining “chemistry-on-the-ligand”, stepwise complexation and “chemistry-on-the-complex” with minimal synthetic efforts. In the final step, Pd-mediated borylation and subsequent Suzuki–Miyaura cross-coupling was employed to introduce the different (multi)donor moieties at the preassembled P–A dyad subunit. The (spectro-)electrochemical data revealed preserved redox properties of the subunits and minimal driving force for oxidative quenching by the naphthalene diimide-based (NDI) acceptor and, thus, high-energy charge separated (CS) states. Time-resolved transient absorption and emission data revealed the formation of long-lived CS states in the polymer-based triads, i.e., the CS lifetime is extended by 2 orders of magnitude in comparison to the molecular triad. The long-lived CS state (13.2 μs) of the conjugated polycarbazole (Carbn) multidonor demonstrates that the rational modular design and efficient synthesis of advanced photoredox-active assemblies can be readily achieved by late-stage diversification utilizing the “chemistry-on-the-complex” approach.
从分子捐献者到聚合物捐献者:模块化光氧化活性 Ru(II) 多吡啶三元组中的长时间电荷分离
在这篇论文中,通过将 "配体化学"、逐步复合和 "复合物化学 "相结合,以最小的合成工作量,介绍了从一种配体前体衍生出的光氧化活性二元、三元和四元的分化模块化合成。在最后一步,利用钯介导的硼酸化和随后的铃木-米亚乌拉交叉偶联,在预组装的 P-A 二元亚基上引入了不同的(多)供体分子。光谱)电化学数据显示,亚基的氧化还原特性得到了保留,萘二亚胺基(NDI)受体的氧化淬灭驱动力最小,因此出现了高能电荷分离(CS)态。时间分辨瞬态吸收和发射数据显示,聚合物三元组中形成了长寿命 CS 态,即 CS 的寿命比分子三元组延长了 2 个数量级。共轭聚咔唑(Carbn)多载体的长寿命 CS 状态(13.2 μs)表明,利用 "复合物上的化学 "方法,通过后期多样化,可以很容易地实现先进光氧化活性组件的合理模块化设计和高效合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


