ACSL4 and polyunsaturated lipids support metastatic extravasation and colonization
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Metastatic dissemination to distant organs demands that cancer cells possess high morphological and metabolic adaptability. However, contributions of the cellular lipidome to metastasis remain elusive. Here, we uncover a correlation between metastasis potential and ferroptosis susceptibility in multiple cancers. Metastases-derived cancer cells exhibited higher ferroptosis sensitivity and polyunsaturated fatty acyl (PUFA)-lipid contents than primary-tumor-derived cells from ovarian cancer patients. Metabolism-focused CRISPR screens in a mouse model for ovarian cancer distant metastasis established via two rounds of in vivo selection revealed the PUFA-lipid biosynthesis enzyme acyl-coenzyme A (CoA) synthetase long-chain family member 4 (ACSL4) as a pro-hematogenous metastasis factor. ACSL4 promotes metastatic extravasation by enhancing membrane fluidity and cellular invasiveness. While promoting metastasis, the high PUFA-lipid state creates dependencies on abhydrolase-domain-containing 6, acylglycerol lipase (ABHD6), enoyl-CoA delta isomerase 1 (ECI1), and enoyl-CoA hydratase 1 (ECH1)—rate-limiting enzymes preparing unsaturated fatty acids (UFAs) for β-oxidation. ACSL4/ECH1 co-inhibition achieved potent suppression of metastasis. Our work establishes the dual functions of PUFA-lipids in tumor progression and metastasis that may be exploitable for therapeutic development.
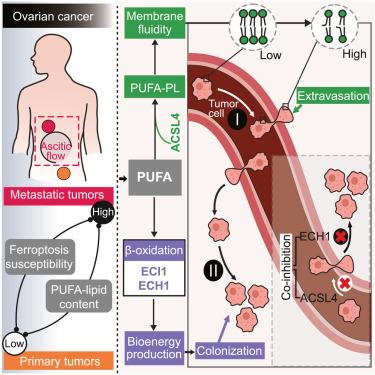
ACSL4 和多不饱和脂质支持转移性外渗和定植
向远处器官转移需要癌细胞具有高度的形态和代谢适应性。然而,细胞脂质体对转移的贡献仍然难以捉摸。在这里,我们发现了多种癌症的转移潜力与铁蛋白沉积易感性之间的相关性。与卵巢癌患者的原发肿瘤衍生细胞相比,转移灶衍生的癌细胞表现出更高的铁变态反应敏感性和多不饱和脂肪酸酰基(PUFA)脂质含量。在通过两轮体内筛选建立的卵巢癌远处转移小鼠模型中,以代谢为重点的CRISPR筛选发现,多不饱和脂肪酸脂质生物合成酶酰辅酶A(CoA)合成酶长链家族成员4(ACSL4)是一种促血源性转移因子。ACSL4通过增强膜流动性和细胞侵袭性来促进转移外渗。在促进转移的同时,高PUFA-脂质状态会对abhydrolase-domain-containing 6、acylglycerol lipase (ABHD6)、enoyl-CoA delta isomerase 1 (ECI1)和enoyl-CoA hydratase 1 (ECH1)--为β-氧化准备不饱和脂肪酸(UFAs)的限速酶产生依赖性。ACSL4/ECH1共同抑制可有效抑制转移。我们的研究确立了 PUFA 脂质在肿瘤进展和转移中的双重功能,可用于治疗开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
文献相关原料
公司名称
产品信息
阿拉丁
Arachidonic acid
阿拉丁
methyl tert-butyl ether
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


