A metagenome-assembled genome inventory for children reveals early-life gut bacteriome and virome dynamics
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Existing microbiota databases are biased toward adult samples, hampering accurate profiling of the infant gut microbiome. Here, we generated a metagenome-assembled genome inventory for children (MAGIC) from a large collection of bulk and viral-like particle-enriched metagenomes from 0 to 7 years of age, encompassing 3,299 prokaryotic and 139,624 viral species-level genomes, 8.5% and 63.9% of which are unique to MAGIC. MAGIC improves early-life microbiome profiling, with the greatest improvement in read mapping observed in Africans. We then identified 54 candidate keystone species, including several Bifidobacterium spp. and four phages, forming guilds that fluctuated in abundance with time. Their abundances were reduced in preterm infants and were associated with childhood allergies. By analyzing the B. longum pangenome, we found evidence of phage-mediated evolution and quorum sensing-related ecological adaptation. Together, the MAGIC database recovers genomes that enable characterization of the dynamics of early-life microbiomes, identification of candidate keystone species, and strain-level study of target species.
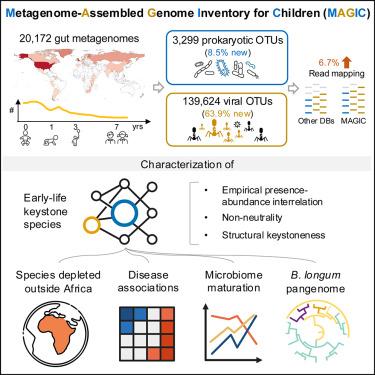
元基因组组装的儿童基因组清单揭示了生命早期肠道细菌组和病毒组的动态变化
现有的微生物群数据库偏重于成人样本,妨碍了对婴儿肠道微生物群的准确分析。在这里,我们从大量富含病毒样颗粒的0到7岁儿童元基因组中生成了元基因组组装的儿童基因组库(MAGIC),其中包括3299个原核生物基因组和139624个病毒物种级基因组,其中8.5%和63.9%是MAGIC所独有的。MAGIC 改进了生命早期微生物组图谱分析,在非洲人中观察到的读图映射改进最大。然后,我们确定了 54 个候选的关键物种,包括几个双歧杆菌属和四个噬菌体,它们组成了丰度随时间波动的行会。它们在早产儿中的丰度降低,并与儿童过敏症有关。通过分析B. longum庞基因组,我们发现了噬菌体介导的进化和与法定量感应相关的生态适应的证据。总之,MAGIC 数据库所恢复的基因组能够描述生命早期微生物群的动态特征、鉴定候选关键物种以及对目标物种进行菌株级研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


