Infraslow noradrenergic locus coeruleus activity fluctuations are gatekeepers of the NREM–REM sleep cycle
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
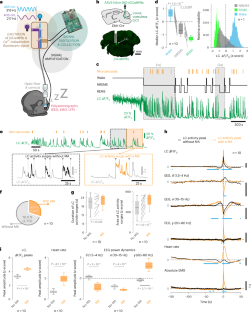
The noradrenergic locus coeruleus (LC) regulates arousal levels during wakefulness, but its role in sleep remains unclear. Here, we show in mice that fluctuating LC neuronal activity partitions non-rapid-eye-movement sleep (NREMS) into two brain–autonomic states that govern the NREMS–REMS cycle over ~50-s periods; high LC activity induces a subcortical–autonomic arousal state that facilitates cortical microarousals, whereas low LC activity is required for NREMS-to-REMS transitions. This functional alternation regulates the duration of the NREMS–REMS cycle by setting permissive windows for REMS entries during undisturbed sleep while limiting these entries to maximally one per ~50-s period during REMS restriction. A stimulus-enriched, stress-promoting wakefulness was associated with longer and shorter levels of high and low LC activity, respectively, during subsequent NREMS, resulting in more microarousal-induced NREMS fragmentation and delayed REMS onset. We conclude that LC activity fluctuations are gatekeepers of the NREMS–REMS cycle and that this role is influenced by adverse wake experiences. Lüthi and colleagues show that activity of the locus coeruleus (LC) is crucial for the cyclic alternation between non-rapid-eye-movement and rapid-eye-movement sleep. Stressful experiences during waking can disrupt LC activity in sleep, which disorganizes the sleep cycle and increases microarousals.

下流去甲肾上腺素能区域小脑活动波动是 NREM-REM 睡眠周期的守门员
去甲肾上腺素能区室(LC)调节清醒时的唤醒水平,但它在睡眠中的作用仍不清楚。在这里,我们在小鼠身上发现,波动的LC神经元活动将非快速眼动睡眠(NREMS)划分为两种大脑-自律神经状态,这两种状态支配着约50秒的NREMS-REMS周期;高LC活动诱导皮层下-自律神经唤醒状态,促进皮层微唤醒,而低LC活动则是NREMS到REMS过渡所必需的。这种功能交替通过在不受干扰的睡眠期间为快速动眼期的进入设置允许的窗口来调节快速动眼期的持续时间,而在快速动眼期受限期间则将这些进入限制在每 50 秒钟最多一次。在随后的 NREMS 期间,刺激丰富、压力促进的唤醒分别与较长和较短的高和低 LC 活动水平相关,从而导致更多由微唤醒引起的 NREMS 破碎和 REMS 开始延迟。我们的结论是,低密度脂蛋白胆碱活动波动是 NREMS-REMS 周期的守门员,而这一作用会受到不良觉醒经历的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


