The potential of targeted radionuclide therapy to treat hypoxic tumor cells
IF 3
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
Abstract
Tumor hypoxia contributes to cancer progression and therapy resistance. Several strategies have been investigated to relieve tumor hypoxia, of which some were successful. However, their clinical application remains challenging and therefore they are not used in daily clinical practice. Here, we review the potential of targeted radionuclide therapy (TRT) to eradicate hypoxic cancer cells. We present an overview of the published TRT strategies using β‐-particles, α-particles, and Auger electrons. Altogether, we conclude that α-particle emitting radionuclides are most promising since they can cause DNA double strand breaks independent of oxygen levels. Future directions for research are addressed, including more adequate in vitro and in vivo models to proof the potential of TRT to eliminate hypoxic cancer cells. Furthermore, dosimetry and radiobiology are identified as key to better understand the mechanism of action and dose-response relationships in hypoxic tumor areas. Finally, we can conclude that in order to achieve long-term anti-tumor efficacy, TRT combination treatment strategies may be necessary.
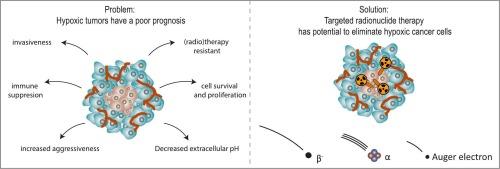
放射性核素靶向疗法治疗缺氧肿瘤细胞的潜力。
肿瘤缺氧会导致癌症进展和耐药性。目前已研究出多种缓解肿瘤缺氧的策略,其中一些获得了成功。然而,它们的临床应用仍然具有挑战性,因此并未用于日常临床实践。在此,我们回顾了放射性核素靶向治疗(TRT)根除缺氧癌细胞的潜力。我们概述了已发表的使用β粒子、α粒子和奥杰电子的TRT策略。总之,我们得出的结论是,发射α粒子的放射性核素最有前途,因为它们可以导致DNA双股断裂,而不受氧气水平的影响。我们还讨论了未来的研究方向,包括建立更充分的体外和体内模型,以证明 TRT 消除缺氧癌细胞的潜力。此外,剂量测定和放射生物学被认为是更好地了解缺氧肿瘤区域的作用机制和剂量-反应关系的关键。最后,我们可以得出结论,为了实现长期的抗肿瘤疗效,TRT 联合治疗策略可能是必要的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


