Salvianolic acid B alleviated myocardial ischemia-reperfusion injury via modulating SIRT3-mediated crosstalk between mitochondrial ROS and NLRP3
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Mitochondrial ROS (mtROS) accumulation and NLRP3 inflammasome activation are critical in the pathogenesis of myocardial ischemia-reperfusion injury (MIRI). However, their upstream regulatory mechanisms and interaction remain inadequately understood.
Purpose
The study aims to investigate the therapeutic effect of Salvianolic acid B (Sal B) on MIRI and elucidate its potential molecular mechanism, mainly focusing on the role of SIRT3.
Methods
SIRT3 was knocked down (SIRT3KD) and overexpressed (SIRT3OE) using small interfering RNA and plasmid, respectively. The role of SIRT3 in the cardioprotective effect of Sal B was explored using MIRI rat models and H9c2 cell hypoxia/reoxygenation (H/R) models. SIRT3, NLRP3 inflammasome proteins, and MnSOD expression were analyzed by Western blot and immunofluorescence staining. MtROS levels were assessed with mitochondrial superoxide indicators (MitoSOX™ Red). ELISA was utilized to measure the levels of LDH, CK-MB, cTnT, and markers of inflammation and oxidative stress. The interaction between SIRT3 and Sal B was studied through biolayer interferometry, cellular thermal shift assay and molecular docking.
Results
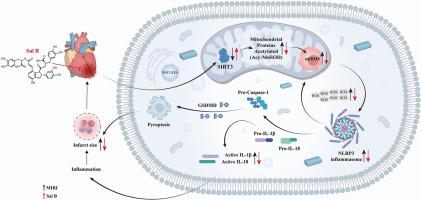
Our findings revealed significantly decreased SIRT3 level, enhanced MnSOD acetylation, and activated NLRP3 inflammasome in myocardium after MIRI and H9c2 cardiomyocytes exposed to H/R conditions. SIRT3KD promoted MnSOD acetylation and NLRP3 expression, aggravating mtROS accumulation and inflammation. Conversely, SIRT3OE significantly inhibited MnSOD acetylation and NLRP3 inflammasome activation. In vitro studies confirmed the crosstalk between mtROS and NLRP3, demonstrating that mtROS scavenger inhibited NLRP3 inflammasome activation induced by H/R and SIRT3KD, and the NLRP3 inhibitor suppressed MnSOD acetylation in H/R and SIRT3KD cardiomyocytes. Interestingly, Sal B was found to bind and upregulate SIRT3, reduce the expression of Acy-MnSOD, NLRP3, ASC, Caspase-1, and GSDMD, inhibit oxidative stress and inflammatory response, decrease myocardial infarct size and ST-segment elevation, and restore myocardial morphology. However, the protective effect of Sal B against MIRI was nullified by a specific SIRT3 inhibitor.
Conclusion
This study unveils that the SIRT3-mediated interplay between mtROS and the NLRP3 inflammasome is pivotal in the pathogenesis of MIRI. Furthermore, it highlights Sal B as a novel therapeutic agent that alleviates MIRI by targeting SIRT3, offering new insights into MIRI treatment.
丹酚酸B通过调节SIRT3介导的线粒体ROS与NLRP3之间的串扰减轻心肌缺血再灌注损伤
背景:线粒体 ROS(mtROS)积累和 NLRP3 炎症小体激活在心肌缺血再灌注损伤(MIRI)的发病机制中至关重要。目的:本研究旨在探讨丹酚酸 B(Sal B)对 MIRI 的治疗作用,并阐明其潜在的分子机制,主要关注 SIRT3 的作用:方法:利用小干扰RNA和质粒分别敲除(SIRT3KD)和过表达(SIRT3OE)SIRT3。利用 MIRI 大鼠模型和 H9c2 细胞缺氧/再氧合(H/R)模型探讨了 SIRT3 在 Sal B 的心脏保护作用中的作用。通过 Western 印迹和免疫荧光染色分析了 SIRT3、NLRP3 炎性体蛋白和 MnSOD 的表达。用线粒体超氧化物指示剂(MitoSOX™ Red)评估MtROS水平。利用酶联免疫吸附法测定 LDH、CK-MB、cTnT 以及炎症和氧化应激标志物的水平。通过生物层干涉测量法、细胞热转移测定法和分子对接法研究了 SIRT3 和 Sal B 之间的相互作用:结果:我们的研究结果表明,MIRI和H9c2心肌细胞暴露于H/R条件后,心肌中的SIRT3水平明显降低,MnSOD乙酰化增强,NLRP3炎性体被激活。SIRT3KD 促进了 MnSOD 乙酰化和 NLRP3 的表达,加剧了 mtROS 的积累和炎症反应。相反,SIRT3OE 能显著抑制 MnSOD 乙酰化和 NLRP3 炎性体的激活。体外研究证实了 mtROS 和 NLRP3 之间的相互影响,表明 mtROS 清除剂抑制了 H/R 和 SIRT3KD 诱导的 NLRP3 炎性体的激活,而 NLRP3 抑制剂抑制了 H/R 和 SIRT3KD 心肌细胞中 MnSOD 的乙酰化。有趣的是,研究发现 Sal B 能结合并上调 SIRT3,减少 Acy-MnSOD、NLRP3、ASC、Caspase-1 和 GSDMD 的表达,抑制氧化应激和炎症反应,缩小心肌梗死面积和 ST 段抬高,恢复心肌形态。然而,Sal B 对 MIRI 的保护作用被特异性 SIRT3 抑制剂所抵消:本研究揭示了 SIRT3 介导的 mtROS 与 NLRP3 炎性体之间的相互作用在 MIRI 的发病机制中起着关键作用。此外,它还强调了 Sal B 是一种新型治疗药物,可通过靶向 SIRT3 缓解 MIRI,为 MIRI 的治疗提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


