Self-catalyzed nitric oxide nanocomplexes induce ferroptosis for cancer immunotherapy
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Ferroptosis, triggered by membrane lipid peroxidation (LPO) and diminished antioxidants, can be induced by intracellular iron (II, Fe2+). However, the role of nitric oxide (NO) in causing Fe2+ overload for ferroptosis remains uncertain. This study reveals that NO can stimulate endogenous Fe2+ release by upregulating heme oxygenase 1 (HMOX1) expression. Here, ferritin heavy chain (FHC) siRNA and hyaluronic acid (HA)-modified Arg-stabilized zinc peroxide (AZOSH), a non-ferrous-based nanoagent, is synthesized to trigger ferroptosis by inducing intracellular Fe2+ overload. AZOSH, a self-catalyzed NO nanocomplex, effectively generates NO through a reaction of self-supplied Arginine (Arg) and hydrogen peroxide (H2O2), which promotes glutathione (GSH) consumption to downregulate glutathione peroxidase 4 (GPX4) expression and produces peroxynitrite (ONOO−) to enhance LPO. Meanwhile, NO promotes endo/lysosomal escape of siRNA by damaging membrane structures. Moreover, AZOSH significantly triggers Fe2+ overload through the synergistic effects of NO-activated HMOX1 expression and FHC siRNA-mediated ferritin sequestration. Additionally, the released Zn2+ from AZOSH induces oxidative stress by inhibiting mitochondrial function, further promoting ferroptosis. Consequently, AZOSH-mediated ferroptosis exhibits a strong cellular immunogenic response for T-cell activation and infiltration. Importantly, the integration of AZOSH with an anti-PD-1 antibody results in notable antitumor efficacy in vivo. Therefore, this study provides a novel concept of NO-induced ferroptosis, highlighting its role in enhancing PD-1-based immunotherapeutic efficacy.
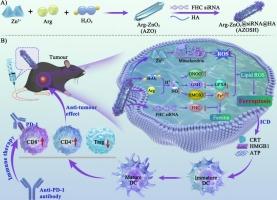
自催化一氧化氮纳米复合物诱导铁氧化酶用于癌症免疫疗法
由膜脂质过氧化(LPO)和抗氧化剂减少引发的铁变态反应可由细胞内铁(II,Fe2+)诱发。然而,一氧化氮(NO)在导致Fe2+超载从而引起铁变态反应中的作用仍不确定。本研究发现,NO 可通过上调血红素加氧酶 1(HMOX1)的表达来刺激内源性 Fe2+ 的释放。在这里,铁蛋白重链(FHC)siRNA与透明质酸(HA)修饰的Arg稳定过氧化锌(AZOSH)(一种非铁基纳米试剂)合成,通过诱导细胞内Fe2+超载来触发铁变态反应。AZOSH 是一种自催化的 NO 纳米复合物,通过自供精氨酸(Arg)和过氧化氢(H2O2)的反应有效地生成 NO,从而促进谷胱甘肽(GSH)的消耗以降低谷胱甘肽过氧化物酶 4(GPX4)的表达,并产生过亚硝酸盐(ONOO-)以增强 LPO。同时,NO 通过破坏膜结构促进 siRNA 的内/溶酶体逃逸。此外,通过 NO 激活的 HMOX1 表达和 FHC siRNA 介导的铁蛋白螯合的协同作用,AZOSH 还能明显引发 Fe2+ 过载。此外,AZOSH 释放的 Zn2+ 通过抑制线粒体功能诱导氧化应激,进一步促进铁蛋白沉积。因此,AZOSH 介导的铁蛋白沉积表现出强烈的细胞免疫原性反应,导致 T 细胞活化和浸润。重要的是,将 AZOSH 与抗 PD-1 抗体结合可在体内产生显著的抗肿瘤疗效。因此,这项研究提供了氮氧化物诱导铁跃迁的新概念,强调了氮氧化物在增强基于 PD-1 的免疫治疗效果中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


