In Silico Inspired Design of Urea Noscapine Congeners as Anticancer Agents: Chemical Synthesis and Experimental Evaluation Using Breast Cancer Cells and a Xenograft Mouse Model
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
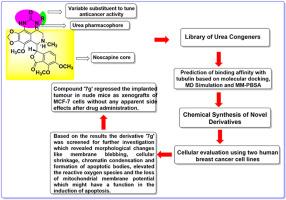
A series of semisynthetic noscapine-urea congeners (7a-7h) as potential tubulin-binding agents are being developed by integrating a urea pharmacophore at the C-9 position of the noscapine scaffold. Their binding affinity to tubulin was predicted through molecular docking, molecular dynamics (MD) simulations, and the MM-PBSA approach. These molecules were subsequently chemically synthesized and assessed using breast cancer cell lines (MCF-7 and MDA-MB-231) and normal human embryonic kidney cells (HEK). Both the docking score and the predicted binding free energy (ΔGbind,pred) revealed that urea congeners had a stronger affinity towards tubulin than noscapine and effectively inhibited the proliferation of all cancer cell types without affecting normal healthy cells. The results indicated that compound 7g exhibited the most promise and was chosen for further studies. Moreover, MDA-MB-231 cells treated with 7g at its IC50 concentration showed morphological changes such as membrane blebbing, fragmented nuclei, and the presence of apoptotic bodies. Apoptosis induction was further confirmed by flow cytometry. Moreover, the tubulin binding assay revealed a greater binding affinity with an equilibrium dissociation constant (KD) of 42 ± 2.4 μM for compound 7g. The number of MCF-7 cells engrafted as breast tumors in nude mice was found to be reduced significantly without any adverse effects. Noscapine is already in clinical trials, but the urea noscapine congener offers an advantage because of its increased potency without impacting the nontoxic profile of noscapine.
在硅学启发下设计作为抗癌剂的尿苷元同系物:利用乳腺癌细胞和异种移植小鼠模型进行化学合成和实验评估
通过在莨菪碱支架的 C-9 位整合脲药源,一系列半合成的莨菪碱-脲同系物(7a-7h)作为潜在的小管蛋白结合剂正在被开发出来。通过分子对接、分子动力学(MD)模拟和 MM-PBSA 方法预测了它们与小管蛋白的结合亲和力。随后对这些分子进行了化学合成,并使用乳腺癌细胞系(MCF-7 和 MDA-MB-231)和正常人类胚胎肾细胞(HEK)进行了评估。对接得分和预测结合自由能(ΔGbind,pred)均显示,脲同系物对小管蛋白的亲和力强于诺卡平,能有效抑制所有癌细胞类型的增殖,而不影响正常健康细胞。结果表明,化合物 7g 最有前景,被选作进一步研究的对象。此外,用 IC50 浓度的 7g 处理的 MDA-MB-231 细胞出现了形态学变化,如膜破裂、细胞核破碎和出现凋亡体。流式细胞术进一步证实了凋亡诱导作用。此外,小管蛋白结合试验显示化合物 7g 具有更高的结合亲和力,其平衡解离常数(KD)为 42 ± 2.4 μM。研究发现,裸鼠体内移植为乳腺肿瘤的 MCF-7 细胞数量明显减少,且无任何不良反应。那可丁已进入临床试验阶段,但尿素那可丁同系物的优势在于其效力的提高而不会影响那可丁的无毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


