Insights into the sustainable design of engineered hydrochar co-doped with cobalt and nitrogen as peroxymonosulfate activator for fluoroquinolones removal
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
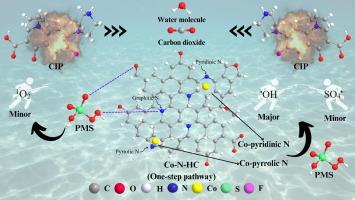
The exploration of waste-derived hydrochar as peroxymonosulfate (PMS) activator for fluoroquinolones removal remains limited. Herein, various Co, N-co-doped hydrochars (Co-N-HCs) were designed via different fabrication pathways (i.e., one-, two-, and three-step pathways) and their characteristics were investigated, revealing the variation in surface chemistry due to different fabrication approaches. These Co-N-HCs with different surface chemistry were employed to remove fluoroquinolone antibiotics, namely ciprofloxacin (CIP), via PMS activation and the results show that the performance of one-step catalyst (HC-1S-A-2) was the highest with kapp = 0.026 min−1 compared to the two-step and three-step catalysts (0.008 – 0.022 min−1). The better performance of the HC-1S-A-2 was due to its highest ID/IG ratio (2.20) and relatively higher electronic conductivity (6.21 × 10-4 S m−1) of the catalyst, which could enhance the PMS activation and reactive species (RS) generation for CIP removal. The catalyst was further optimized by varying Co content, and the 2.0 wt% Co content (HC-1S-A-3) emerged as the most effective, demonstrating efficient CIP removal across various operational conditions. The chemical scavenging and electrochemical studies revealed that the hydroxyl radical (major ROS), sulfate radical, singlet oxygen, and other nonradical pathways were involved in CIP degradation while the major active site was Co coupled with pyrrolic N and pyridinic N. Additionally, based on the identified CIP intermediates during the degradation process, the CIP degradation pathways were proposed, and the intermediates were subjected to a toxicity assessment. The results showed that CIP and its intermediates can be successfully mineralized and detoxified by increasing the catalytic reaction time. Overall, this work provides a sustainable approach to transform waste into engineered hydrochar for pollutants removal.
掺杂钴和氮的工程炭作为过硫酸盐活化剂去除氟喹诺酮类药物的可持续设计见解
将废物衍生水煤浆作为过一硫酸盐(PMS)活化剂用于去除氟喹诺酮类药物的探索仍然有限。本文通过不同的制备途径(即一步法、二步法和三步法)设计了各种掺杂 Co、N 的水碳(Co-N-HCs),并研究了它们的特性,揭示了不同制备方法导致的表面化学变化。结果表明,与两步法和三步法催化剂(0.008 - 0.022 min-1)相比,一步法催化剂(HC-1S-A-2)的性能最高,kapp = 0.026 min-1。HC-1S-A-2 催化剂性能更好的原因在于其最高的内径/内径比(2.20)和相对较高的电子电导率(6.21 × 10-4 S m-1),这可以增强 PMS 的活化和反应物(RS)的生成,从而去除 CIP。通过改变 Co 含量对催化剂进行了进一步优化,2.0 wt% Co 含量(HC-1S-A-3)的催化剂最为有效,在各种操作条件下都能高效去除 CIP。化学清除和电化学研究表明,羟基自由基(主要的 ROS)、硫酸根自由基、单线态氧和其他非自由基途径参与了 CIP 降解,而主要的活性位点是 Co 与吡咯烷 N 和吡啶 N 的偶联。结果表明,通过增加催化反应时间,CIP 及其中间产物可以成功矿化和解毒。总之,这项工作提供了一种可持续的方法,将废物转化为工程水炭,用于去除污染物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


