Enhancement of CuBr-based catalysts for aerobic alcohol oxidation enabled by rational design of bifunctional ligands featuring both a N-alkyl substituted ethylenediamine skeleton and a TEMPO moiety
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The development of a simple and highly efficient catalytic system for the selective oxidation of alcohols in open air is an extremely important but challenging task in the fields of organic chemistry and catalysis. In this study, three novel bifunctional ligands, named L1-L3, were designed by combining a N-alkyl substituted ethylenediamine skeleton with a TEMPO moiety varying the length of the linker between the two components. When paired with CuBr, ligand L2, which contains six methylene groups in the linker, demonstrated excellent catalytic activity in the aerobic oxidation of benzyl alcohol, achieving a quantitative yield under ambient conditions. Moreover, the developed CuBr/L2 catalytic system exhibited a broad substrate scope, including primary benzylic, heterocyclic, allylic, aliphatic alcohols, and secondary benzylic alcohols. Mechanistic insights were gained using cyclic voltammetry (CV), ultraviolet–visible (UV–vis) spectroscopy, and electron spray ionization mass (ESI-MS) spectrometry, enabling stepwise monitoring of the reaction. These studies revealed that the mono-copper species is a key intermediate, with its oxidation state cycling between Cu(I) and Cu(II), playing a pivotal role in the aerobic oxidation of alcohols. Additionally, the moderate linker length in ligand L2 facilitated internal interaction between the TEMPO moiety and the copper center, thereby enhancing catalytic activity with a high turnover frequency (TOF) of 33 h−1.
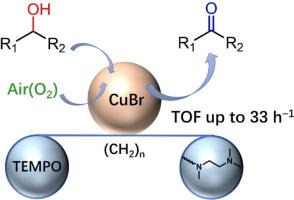
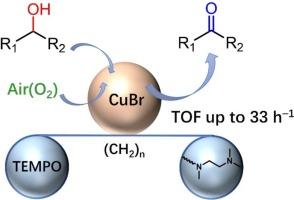
通过合理设计具有 N-烷基取代乙二胺骨架和 TEMPO 分子的双功能配体,增强铜硼基催化剂的有氧醇氧化性能
在有机化学和催化领域,开发一种简单而高效的催化系统来实现露天醇的选择性氧化是一项极其重要但又极具挑战性的任务。本研究设计了三种新型双功能配体(命名为 L1-L3),将 N-烷基取代的乙二胺骨架与 TEMPO 分子结合在一起,并改变两个组分之间连接物的长度。配体 L2 与 CuBr 配对后,在有氧氧化苯甲醇的过程中表现出优异的催化活性,在环境条件下实现了定量产率。此外,所开发的 CuBr/L2 催化体系具有广泛的底物范围,包括伯苄基醇、杂环醇、烯丙基醇、脂肪醇和仲苄基醇。利用循环伏安法(CV)、紫外-可见光谱法(UV-vis)和电子喷雾电离质谱法(ESI-MS)对反应进行了逐步监测,从而获得了对机理的深入了解。这些研究表明,单铜物种是一种关键的中间体,其氧化态在 Cu(I)和 Cu(II)之间循环,在醇的有氧氧化过程中起着关键作用。此外,配体 L2 中的连接体长度适中,有利于 TEMPO 分子与铜中心之间的内部相互作用,从而提高了催化活性,其周转频率(TOF)高达 33 h-1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


