Design and synthesis of novel derivatives of bisepoxylignans as potent anti-inflammatory agents involves the modulation of the M1/M2 microglia phenotype via TLR4/NF-κB signaling pathway
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Bisepoxylignans have been reported to possess a variety of biological functions, especially in anti-inflammatory aspects. However, the bis-tetrahydrofuran scaffold restricts the type and position of substituents, which further limits the further optimization of their biological activity and druggability. Here, a series of novel derivative s of bisepoxylignans bearing 7H-pyrrolo[2,3-d]pyrimidin-4-amine and 1H-pyrazolo[3,4-d]pyrimidin-4-amine scaffolds were designed and synthesized by a scaffold hopping strategy. Biological evaluation demonstrated that compound 7x exhibited the most potent anti-inflammatory activity, both in vitro and in vivo. Additionally, 7x displayed an excellent oral safety profile at a dose of 500 mg/kg. The anti-inflammatory effect of 7x is potentially mediated by the inhibition of the TLR4/NF-κB pathway and the promotion of M1 to M2 microglial phenotypic conversion. Taken together, 7x could be a promising lead compound for the development of novel therapeutic agents for the treatment of inflammatory diseases.
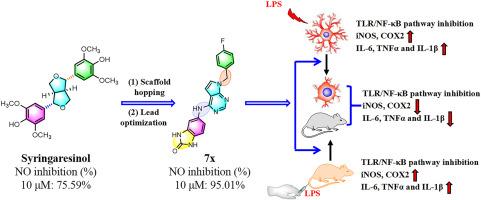
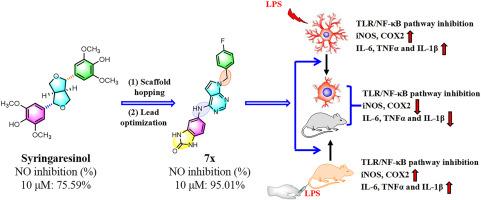
通过TLR4/NF-κB信号通路调节M1/M2小胶质细胞表型,设计和合成新型双环氧木脂素衍生物作为强效抗炎剂
据报道,双环氧木质素具有多种生物功能,尤其是在抗炎方面。然而,双四氢呋喃支架限制了取代基的类型和位置,这进一步限制了其生物活性和可药用性的进一步优化。在此,我们采用支架跳跃策略设计并合成了一系列带有 7H-吡咯并[2,3-d]嘧啶-4-胺和 1H-吡唑并[3,4-d]嘧啶-4-胺支架的双环氧木质素的新型衍生物。生物学评价结果表明,化合物 7x 在体外和体内均表现出最强的抗炎活性。此外,7x 在单次 500 毫克/千克的高剂量下显示出极佳的口服安全性。7x 的抗炎作用可能是通过抑制 TLR4/NF-κB 通路和促进 M1 到 M2 的小胶质细胞表型转换来实现的。综上所述,7x 可能是一种很有前景的先导化合物,可用于开发治疗炎症性疾病的新型治疗剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


