Spectral Fingerprints of DOM-Tungsten Interactions: Linking Molecular Binding to Conformational Changes
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Tungsten (W), a widely used yet understudied emerging contaminant, forms oxyanions in aqueous environments, distinguishing it from conventional heavy metals. While dissolved organic matter (DOM) demonstrates considerable potential for W binding, DOM-W interactions remain largely unexplored. Of particular significance, yet frequently overlooked, are the conformational changes in DOM during W binding processes. This study proposes a novel theoretical framework integrating superposition and charge transfer models to elucidate the complexity of these interactions. By combining spectroscopic techniques and photophysical models, we revealed that aromatic compounds containing 1–3 rings, especially monocyclic aromatic protein-like components, exhibit high affinity for W (logK=3.74–4.00). Phenolic hydroxyls served as primary binding sites for W, with aromatic rings facilitating binding through π interactions. Importantly, W binding to aromatic compounds induced conformational changes in DOM, transitioning from a loosely aggregated state to a more compact configuration. These changes facilitated W encapsulation within DOM through the synergistic effects of hydrophobic interactions, hydrogen/π-hydrogen bonding and π-stacking, potentially leading to stable trapping of W. Two-dimensional correlation spectroscopy analysis elucidated the sequential encapsulation process, involving phenolic, aromatic carboxylic/aliphatic carboxylic, polysaccharides, and aliphatics. The intricate behavior of DOM-W binding profoundly reshapes DOM's conformation, subtly yet significantly orchestrating W's binding affinity, environmental transport, and bioavailability in aquatic ecosystems.
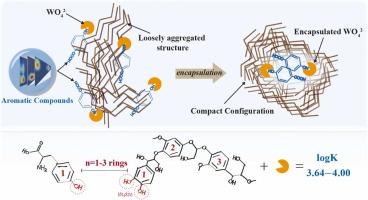
DOM 与钨相互作用的光谱指纹:将分子结合与构象变化联系起来
钨(W)是一种广泛使用但研究不足的新兴污染物,它在水环境中形成氧阴离子,有别于传统的重金属。虽然溶解有机物(DOM)显示出与钨结合的巨大潜力,但 DOM 与钨的相互作用在很大程度上仍未得到探索。在 W 结合过程中,DOM 的构象变化具有特别重要的意义,但却经常被忽视。本研究提出了一种整合叠加和电荷转移模型的新型理论框架,以阐明这些相互作用的复杂性。通过结合光谱技术和光物理模型,我们发现含有 1-3 个环的芳香族化合物,尤其是单环芳香族蛋白样成分,对 W 具有很高的亲和力(logK=3.74-4.00)。酚羟基是 W 的主要结合位点,芳香环通过 π 相互作用促进结合。重要的是,W 与芳香化合物的结合会诱导 DOM 发生构象变化,从松散的聚集状态过渡到更紧凑的构型。这些变化通过疏水相互作用、氢/π-氢键和π-堆叠的协同作用,促进了 W 在 DOM 中的封装,从而可能导致 W 的稳定捕获。二维相关光谱分析阐明了涉及酚类、芳香族羧酸类/脂肪族羧酸类、多糖和脂肪族化合物的顺序封装过程。DOM-W 结合的复杂行为深刻地重塑了 DOM 的构象,微妙而显著地协调了 W 在水生生态系统中的结合亲和力、环境迁移和生物利用率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


