Molecular composition and formation mechanism of chlorinated organic compounds in biological waste leachate treated by electrochemical oxidation with a boron-doped diamond anode
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The use of electrochemical oxidation with boron-doped diamond (BDD) as an anode has been demonstrated to be an effective means of removing dissolved organic matter (DOM) from biologically treated waste leachate. However, in the presence of chloride ions, undesired chlorine evolution occurs on the anode; this forms chlorinated DOM, mostly of unknown molecular composition. We investigate the molecular composition and formation mechanism of chlorinated DOM during electrochemical oxidation process of biologically treated leachate DOM. At a current density of 8 mA/cm2, after 120 min of electrolysis, 479 unknown chlorinated DOMs were detected in the treated effluent, comprising 21.55% of the total. The unknown species are dominated by oxygen-rich, highly unsaturated structures, and exhibit higher oxidation degrees, lower unsaturation, and lower aromaticity compared to the removed nonchlorinated DOM. An additional 43.63 mg/L of known chlorinated DOM species, predominantly dichloroacetic and trichloroacetic acids, also accumulate in the treated effluent. Introducing hydroxyl radicals (HO•) to the anode surface forms reactive chlorine species including chlorine radical (Cl•), dichlorine radical (Cl2•−), and hypochlorous acid/hypochlorite (HOCl/OCl−); the concentration of HOCl/OCl− reaches 529.2 mg/L. These species react with reduced and aromatic dissolved organic matter via reaction pathways such as chlorine substitution for hydrogen (Cl+H-) and the HOCl addition reaction (HO+Cl+) to generate unknown chlorinated DOM species; the known chlorinated DOM are formed afterward via ring opening and dealkylation pathways. Our results provide a theory for the prevention and control of chlorinated DOM during treatment of chlorine-laden organic wastewater by an electrochemical oxidation system with a boron-doped diamond anode.
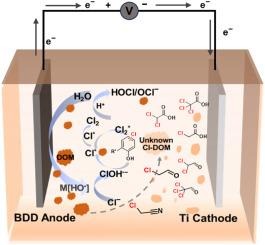
用掺硼金刚石阳极进行电化学氧化处理的生物垃圾渗滤液中氯化有机化合物的分子组成和形成机理。
使用掺硼金刚石(BDD)作为阳极进行电化学氧化,已被证明是去除生物处理废物沥滤液中溶解有机物(DOM)的有效方法。然而,在存在氯离子的情况下,阳极上会发生不希望发生的氯演变;这就形成了氯化 DOM,其中大部分分子成分不明。我们研究了生物处理渗滤液 DOM 电化学氧化过程中氯化 DOM 的分子组成和形成机理。在电流密度为 8 mA/cm2 时,电解 120 分钟后,在处理后的污水中检测到 479 种未知氯化 DOM,占总量的 21.55%。与去除的非氯化 DOM 相比,这些未知物种以富氧、高度不饱和结构为主,并表现出较高的氧化度、较低的不饱和度和较低的芳香度。此外,经处理的污水中还积累了 43.63 毫克/升的已知氯化 DOM 物种,主要是二氯乙酸和三氯乙酸。阳极表面引入羟基自由基 (HO-) 会形成活性氯物种,包括氯自由基 (Cl-)、二氯自由基 (Cl2--) 和次氯酸/次氯酸盐 (HOCl/OCl-);HOCl/OCl- 的浓度达到 529.2 毫克/升。这些物种通过氯代氢(Cl+H-)和 HOCl 加成反应(HO+Cl+)等反应途径与还原型和芳香型溶解有机物发生反应,生成未知的氯化 DOM 物种;之后通过开环和脱烷基化途径形成已知的氯化 DOM。我们的研究结果为利用掺硼金刚石阳极的电化学氧化系统处理含氯有机废水时预防和控制氯化 DOM 提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


