Responsive plasmonic hybrid nanorods enables metabolism reprogramming via cuproptosis-photothermal combined cancer therapy
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Abnormal tumor metabolism leads to tumor growth, metastasis, and recurrence, reprogramming tumor metabolism and activating potent anti-tumor immune response have been demonstrated to have good therapeutic effects on tumor elimination. Copper-based nanomaterials involved in cuproptosis show great prospects in these two aspects, but their efficiency is restricted by Cu homeostasis and the toxicity of the chelator. Here, the pH-responsive AuNRs@Cu2O core-shell plasmonic hybrid nanorods (ACNRs) have been successfully fabricated to realize microenvironment-controlled release at the tumor site for the combined therapy of cuproptosis and photothermal treatment. The AuNRs core exhibited excellent NIR-II photothermal property, which boost the intracellular concentration of copper to trigger severe cuproptosis and induce immunogenic cell death of tumor cells. In vivo studies demonstrated the ACNR exhibited efficient tumor therapy for primary, metastatic, and recurrent tumors. ACNRs-induced cuproptosis and PTT were capable of reprogramming energy metabolism, leading to a decreased production of lactic acid. This potential of metabolic reprogramming assisted in reshaping the immunosuppressive tumor microenvironment to facilitate the infiltration of immune cells and boost the immune responses triggered by PTT. The therapeutic mechanism was further verified by metabolomics analysis, which indicated that ACNRs + PTT treatment led to the inhibition of the Pentose Phosphate Pathway and Glycolysis pathways in tumor cells. The suppression of glycolytic reduced ATP synthesis, thereby hindering energy-dependent copper efflux, which in turn promoted cuproptosis. Taken together, this study offers promising insights for cuproptosis-based cancer treatment and sheds new light on nanomedicine-mediated metabolic modulation for future tumor therapy.
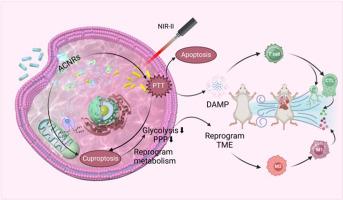
响应性等离子杂化纳米棒通过杯突-光热联合癌症疗法实现新陈代谢重编程。
肿瘤代谢异常会导致肿瘤生长、转移和复发,重编程肿瘤代谢和激活强效抗肿瘤免疫反应已被证明对消除肿瘤具有良好的治疗效果。铜基纳米材料参与铜跃迁在这两方面显示出巨大的前景,但其效率受到铜平衡和螯合剂毒性的限制。本文成功制备了pH响应的AuNRs@Cu2O核壳质子杂化纳米棒(ACNRs),实现了肿瘤部位的微环境控制释放,用于杯突疗法和光热疗法的联合治疗。AuNRs 内核表现出优异的近红外-II 光热特性,可提高铜在细胞内的浓度,引发严重的杯突症,诱导肿瘤细胞免疫性死亡。体内研究表明,AcNR 能有效治疗原发性、转移性和复发性肿瘤。ACNR 诱导的杯突症和 PTT 能够重新规划能量代谢,从而减少乳酸的产生。这种代谢重编程的潜力有助于重塑免疫抑制性肿瘤微环境,从而促进免疫细胞的浸润,并增强 PTT 引发的免疫反应。代谢组学分析进一步验证了这一治疗机制,结果表明 ACNRs + PTT 治疗可抑制肿瘤细胞中的磷酸戊糖途径和糖酵解途径。糖酵解的抑制减少了 ATP 的合成,从而阻碍了能量依赖性铜外流,进而促进了杯突症。综上所述,这项研究为基于杯突症的癌症治疗提供了前景广阔的见解,并为纳米药物介导的代谢调节在未来肿瘤治疗中的应用提供了新的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


