Remolding probiotics for effective treatment of type 2 diabetes via oral administration
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Effective, user-friendly, lifestyle-compatible, and economic treatment for type 2 diabetes (T2D) is urgently needed due to its high incidence and health threats. Here, we remolded Lactococcus lactis through genetic engineering to persistently secrete glucagon-like peptide-1 (L. lactis-GLP-1) and subsequent bioorthogonal arming with dopamine (DA)-based “gripper” and β-glucan (GN)-based “shield” (L. lactis-GLP-1-DA@GN) for treatment of T2D mice via oral administration. With protection by GN-based “shield”, L. lactis-GLP-1-DA@GN achieved an impressive enhancement of survival by 20666 times compared with bare L. lactis-GLP-1 after experiencing gastrointestinal conditions and DA-based “gripper” was shielded from interaction with the upper digestive tract. Once prebiotic GN was metabolized by gut microbiota into short-chain fatty acids (SCFAs), underlying DA-based “gripper” was exposed to assist intestinal colonization of L. lactis-GLP-1, achieving synergistic treatment effects through secreted GLP-1 and SCFAs. With all advances, oral administration of L. lactis-GLP-1-DA@GN realized effective T2D treatment through improving glucose/lipid homeostasis, repairing major organs' damages, and positively modulating gut microbiota. Moreover, multi-omics analysis revealed that L. lactis-GLP-1-DA@GN also mainly intervened in liver's signaling pathways regarding lipid metabolism and oxidative regulation to advance anti-T2D process. Our strategy marks reconstruction of probiotics by combining chemical and biological tools, broadening the avenue of manipulating probiotics for disease treatments.
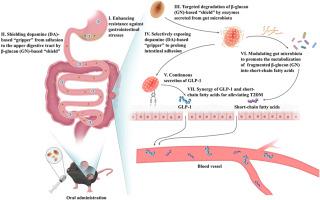
重塑益生菌,通过口服有效治疗 2 型糖尿病。
由于 2 型糖尿病(T2D)的高发病率和对健康的威胁,迫切需要有效、方便、符合生活方式且经济的治疗方法。在这里,我们通过基因工程重塑了乳酸乳球菌,使其能持续分泌胰高血糖素样肽-1(L. lactis-GLP-1),并在其后与基于多巴胺(DA)的 "抓手 "和基于β-葡聚糖(GN)的 "盾牌 "进行生物正交武装(L. lactis-GLP-1-DA@GN),通过口服给药治疗T2D小鼠。在基于 GN 的 "盾牌 "的保护下,L. lactis-GLP-1-DA@GN 在经历胃肠道状况后的存活率比裸 L. lactis-GLP-1 提高了 20666 倍,而基于 DA 的 "抓手 "则避免了与上消化道的相互作用。一旦益生菌 GN 被肠道微生物群代谢为短链脂肪酸(SCFAs),基于 DA 的底层 "抓手 "就会暴露出来,协助 L. lactis-GLP-1 在肠道定植,通过分泌 GLP-1 和 SCFAs 达到协同治疗效果。通过这些进展,口服 L. lactis-GLP-1-DA@GN通过改善血糖/血脂平衡、修复主要器官损伤和积极调节肠道微生物群,实现了对 T2D 的有效治疗。此外,多组学分析表明,L. lactis-GLP-1-DA@GN还主要干预肝脏脂质代谢和氧化调节的信号通路,从而推进抗T2D进程。我们的研究策略标志着通过结合化学和生物学工具重建了益生菌,拓宽了利用益生菌治疗疾病的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


