Si-doped NASICON-type Li1.4Al0.4Ti1.6(PO4)3 solid electrolytes for enhanced stability and performance of Li-CO2 batteries
IF 5.8
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Li-CO2 batteries (LCBs) have attracted significant research interest owing to their potential as energy storage devices and their contribution to carbon neutrality. In this study, we synthesized a solid electrolyte using Si-doped Li1.4Al0.4Ti1.6(PO4)3 (LASTP), by incorporating Si into the NASICON-structured LATP. Through Si doping, P in the tetrahedral PO4 units within the NASICON framework is substituted with Si, and bridging oxygen bonds are formed after high-temperature heat treatment The LASTP powder synthesized via a solution-based method exhibited uniform particle size and composition, and the resulting pellet achieved high densification and the formation of interconnected structures. The pellet exhibited an ionic conductivity of approximately 8.8 × 10-4 S/cm at 25℃. The LCB utilizing LASTP demonstrated a maximum discharge capacity of 23,887 mAh/g and successfully operated for 200 cycles at a current density of 100 mA/g with a cut-off capacity of 500 mAh/g. The post-cycling analysis of the cathode confirmed the reversible reactions of the LCB. Additionally, comparative post-cycling XPS analysis of LATP and LASTP revealed that Si doping in LASTP mitigated the reduction of Ti4+ to Ti3+, thereby enhancing the chemical stability of the solid electrolyte. Also, the structural stability of the solid electrolyte was enhanced owing to the formation of new bonds, surpassing the cycle performance and full-depth capacity of LCBs using conventional solid electrolytes. The introduction of structurally and chemically stabilized LASTP enabled the realization of long-lasting, high-capacity LCBs.
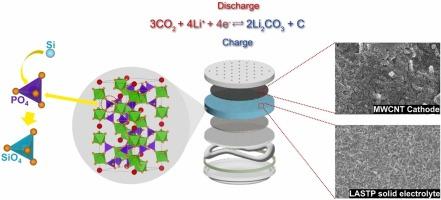
掺杂硅的 NASICON 型 Li1.4Al0.4Ti1.6(PO4)3 固体电解质可提高锂-二氧化碳电池的稳定性和性能
锂-二氧化碳电池(LCB)因其作为储能设备的潜力及其对碳中和的贡献而引起了广泛的研究兴趣。在本研究中,我们通过在 NASICON 结构的 LATP 中掺入硅,合成了一种使用硅掺杂 Li1.4Al0.4Ti1.6(PO4)3 (LASTP) 的固体电解质。通过掺入硅,NASICON 框架内四面体 PO4 单元中的 P 被硅取代,并在高温热处理后形成桥接氧键。 通过溶液法合成的 LASTP 粉末具有均匀的粒度和成分,所得到的颗粒实现了高致密化并形成了相互连接的结构。颗粒在 25℃时的离子导电率约为 8.8 × 10-4 S/cm。利用 LASTP 的 LCB 显示出 23,887 mAh/g 的最大放电容量,并在 100 mA/g 的电流密度下成功运行了 200 个循环,截止容量为 500 mAh/g。阴极的循环后分析证实了 LCB 的可逆反应。此外,对 LATP 和 LASTP 进行的循环后 XPS 比较分析表明,LASTP 中的硅掺杂减轻了 Ti4+ 向 Ti3+ 的还原,从而提高了固体电解质的化学稳定性。此外,由于形成了新的键,固体电解质的结构稳定性也得到了增强,从而超越了使用传统固体电解质的 LCB 的循环性能和全深度容量。通过引入结构和化学稳定的 LASTP,实现了长寿命、高容量低浓电池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


