Coordinated Anions Tune Z-Type Ligand Displacement from Colloidal CdSe and InP Nanocrystal Surfaces
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Neutral metal salts coordinate to the surfaces of colloidal semiconductor nanocrystals (NCs) by acting as Lewis acid acceptors for the NC surface anions. This ligand coordination has been associated with increased emission due to the passivation of surface hole traps. Here, variation of the anionic ligands of metal salts is used to study anion effects on metal complex Lewis acidity and surface coordination at CdSe and InP NCs. To resolve dynamic ligand exchange processes, the tetracarbonylcobaltate anion, [Co(CO)4]−, is used as a monoanionic ligand for which IR spectroscopy can readily identify displacement of neutral M[Co(CO)4]x species (M = Cd or In; x = 2 or 3, respectively) upon addition of neutral donor ligands. Notably, although Cd[Co(CO)4]2 is more Lewis acidic than cadmium oleate, the former is more readily displaced from the NC surfaces. Lewis acidity and X-type anion exchange are, therefore, factors to be considered when performing postsynthetic addition of metal salts for NC photoluminescence emission enhancement.
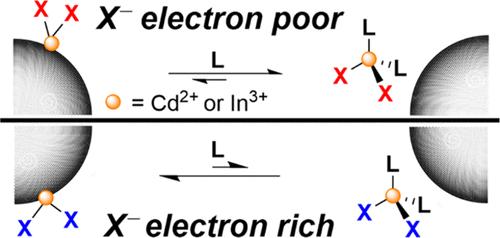
配位阴离子调节 Z 型配体在胶体硒化镉和磷化铟纳米晶表面的位移
中性金属盐通过充当 NC 表面阴离子的路易斯酸受体,与胶体半导体纳米晶体(NC)表面配位。由于表面空穴陷阱的钝化,这种配体配位与发射的增加有关。在此,我们利用金属盐阴离子配体的变化来研究阴离子对 CdSe 和 InP NC 金属复合物路易斯酸度和表面配位的影响。为了解析动态配体交换过程,[Co(CO)4]- 四羰基钴酸盐阴离子被用作单阴离子配体,红外光谱分析可以很容易地识别中性 M[Co(CO)4]x 物种(M = Cd 或 In;x = 2 或 3,分别为中性供体配体)在加入中性配体后的位移。值得注意的是,尽管镉[Co(CO)4]2 比油酸镉更具路易斯酸性,但前者更容易从 NC 表面移出。因此,在合成后添加金属盐以增强 NC 的光致发光时,路易斯酸性和 X 型阴离子交换是需要考虑的因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


