Therapeutic drug monitoring of methotrexate by disposable SPCE biosensor for personalized medicine
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
Methotrexate (MTX) is widely used in clinical practice for the treatment of malignant tumors and autoimmune diseases. High-dose MTX has been shown to be an effective approach for treating various malignant tumors, but it is accompanied by numerous toxic side effects, necessitating therapeutic drug monitoring (TDM) for patients and timely “folinic acid rescue.” High-performance liquid chromatography and fluorescent immunoassay (FIA) are currently used to detect MTX, but these methods are limited by complex sample preparation, time consumption, and high cost. Therefore, a simple, rapid, and cost-effective MTX measurement method is required.
Results
We developed a flexible and inexpensive electrochemical sensor using a stearyl trimethyl ammonium bromide (STAB)-modified, screen-printed carbon electrode to directly detect MTX in human serum. Assay performance was validated via detection of MTX in spiked buffer. The sensor was capable of measuring MTX concentrations ranging from 0.01 to 1 μM and 1–1500 μM, with a limit of detection of 3.1 nM and a limit of quantitation of 3.5 nM. For the samples simulating combined medication, the sensor exhibited outstanding selectivity, in cross-reactivity, with the maximum response value of interferents reaching only 3.49 %. Additionally, the sensor shows reliable repeatability with a relative standard deviation of 3.8 % and remarkable stability. Recovery in human serum validated the clinical utility of the sensor in point-of-care testing conditions. The sensor's applicability to personal medicine was confirmed by detecting MTX blood concentration in patients with different diseases. The results obtained by the sensor were compared with those obtained by the FIA technique, a method commonly used in hospitals, showing a high level of consistency between both methods.
Significance
To meet the requirements of personalized medicine for MTX-patients, we developed a disposable biosensor with wide detection range from 0.01 to 1 μM and 1–1500 μM. Owing to the effective enrichment of STAB, the electrochemical response was sensitive, selective, stable, and rapid. As per the clinical test results, our sensor has shown a high level of consistency with the FIA method, indicating its potential to replace FIA as a cost-effective platform for MTX-TDM.
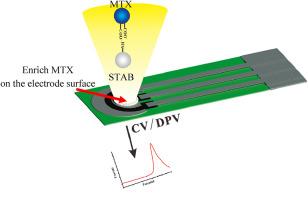
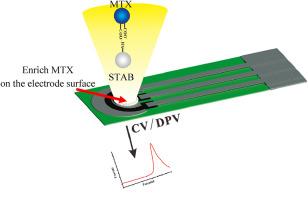
利用一次性 SPCE 生物传感器监测甲氨蝶呤的治疗药物,实现个性化医疗
背景甲氨蝶呤(MTX)在临床上广泛用于治疗恶性肿瘤和自身免疫性疾病。大剂量MTX已被证明是治疗各种恶性肿瘤的有效方法,但它也伴随着许多毒副作用,需要对患者进行治疗药物监测(TDM)并及时进行 "亚叶酸救援"。目前,高效液相色谱法和荧光免疫分析法(FIA)被用于检测 MTX,但这些方法因样品制备复杂、耗时长、成本高而受到限制。因此,我们需要一种简单、快速且经济有效的 MTX 检测方法。结果 我们开发了一种灵活且廉价的电化学传感器,该传感器采用硬脂基三甲基溴化铵(STAB)修饰的丝网印刷碳电极,可直接检测人血清中的 MTX。通过检测加标缓冲液中的 MTX,验证了测定性能。传感器能够测量 0.01-1 μM 和 1-1500 μM 的 MTX 浓度,检测限为 3.1 nM,定量限为 3.5 nM。对于模拟联合用药的样品,该传感器在交叉反应中表现出卓越的选择性,干扰物的最大反应值仅为 3.49%。此外,该传感器还具有可靠的重复性(相对标准偏差为 3.8%)和出色的稳定性。在人体血清中的恢复验证了该传感器在护理点检测条件下的临床实用性。通过检测不同疾病患者血液中的 MTX 浓度,证实了该传感器在个人医疗方面的适用性。为了满足 MTX 患者个性化医疗的要求,我们开发了一种一次性生物传感器,其检测范围从 0.01-1 μM 到 1-1500 μM。由于 STAB 的有效富集,电化学反应灵敏、选择性强、稳定且快速。根据临床测试结果,我们的传感器与 FIA 方法具有高度的一致性,这表明它有望取代 FIA 成为 MTX-TDM 的经济高效平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


