Synthesis of magnetically recyclable FeCeO nanocrystals with heterojunction between Fe2O3 and CeO2 via one-step method toward efficient organic dyes degradation by activating peroxymonosulfate
IF 9.7
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Azo dye wastewater poses a serious threat to environment and human health due to steady structures make them resistant to biodegradation or chemical degradation. The coupling of photocatalysis and peroxymonosulfate (PMS) as an efficient strategy can achieve dye-contaminated water purification by inducing advance oxidation processes. In this study, magnetic FeCeO nanocrystals with heterojunction between Fe2O3 and CeO2 by one-step method were successfully synthesized to efficiently degrade methyl orange (MO) and Congo red (CR) by activating PMS under visible light irradiation. PMS molecules could be adsorbed by Fe2Ce1O composite and generated radicals during photocatalytic process. Free radical quenching experiments and electron paramagnetic resonance (EPR) revealed that radicals were the dominant species in photodegradation process. Fe2Ce1O catalyst could completely decompose dye molecules for waste solutions with low dye concentration. Increasing MO and CR concentrations to 100 mg L-1, the removal efficiencies of MO and CR by Fe2Ce1O were still up to 85.2% and 77.9% in sequence. And liquid chromatography mass spectrometry (LC-MS) was used to confirm the intermediates and degradation pathways during MO and CR degradation processes. Cyclic experiments demonstrated that Fe2Ce1O composite had good durability and reusability. Moreover, degradation mechanisms were investigated. This study provides new insight into the synthesis of heterogeneous metal oxide catalyst to solve the organic pollution issues.
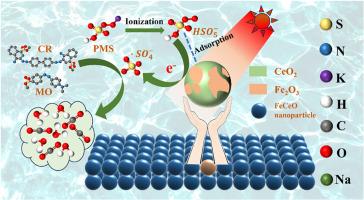
一步法合成具有磁性可回收性的 FeCeO 纳米晶体(Fe2O3 和 CeO2 异质结),通过活化过氧单硫酸盐高效降解有机染料
偶氮染料废水对环境和人类健康构成严重威胁,因为其稳定的结构使其无法被生物降解或化学降解。光催化与过氧化单硫酸盐(PMS)的耦合作为一种有效的策略,可以通过诱导提前氧化过程实现染料污染水的净化。本研究采用一步法成功合成了Fe2O3和CeO2异质结的磁性FeCeO纳米晶体,在可见光照射下通过激活PMS高效降解甲基橙(MO)和刚果红(CR)。PMS 分子可被 Fe2Ce1O 复合材料吸附,并在光催化过程中产生自由基。自由基淬灭实验和电子顺磁共振(EPR)显示,自由基是光降解过程中的主要物种。对于染料浓度较低的废液,Fe2Ce1O 催化剂可以完全分解染料分子。将 MO 和 CR 的浓度提高到 100 mg L-1 后,Fe2Ce1O 对 MO 和 CR 的去除率仍依次高达 85.2% 和 77.9%。液相色谱质谱法(LC-MS)证实了 MO 和 CR 降解过程中的中间产物和降解途径。循环实验表明,Fe2Ce1O 复合材料具有良好的耐久性和可重复使用性。此外,还研究了降解机制。这项研究为合成异质金属氧化物催化剂以解决有机污染问题提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


