Ultrahigh Surface Area Nanoporous Carbons Synthesized via Hypergolic and Activation Reactions for Enhanced CO2 Capacity and Volumetric Energy Density
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
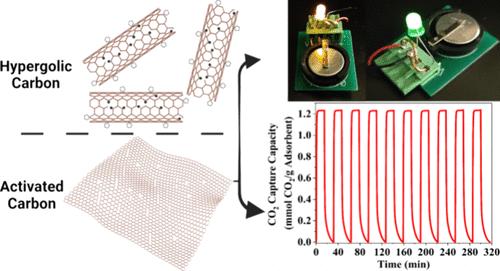
We report a family of carbon sorbents synthesized by integrating hypergolics with activation reactions on a templated substrate. The materials design leads to nanoporous carbons with a BET area of 4800 m2 g–1 with an impressive total pore volume of 2.7 cm3 g–1. To the best of our knowledge, this BET area value is the highest reported in the literature. Electron spin resonance (ESR) measurements determined the number of radicals in an effort to provide a mechanistic understanding of the formation of ultrahigh surface area carbons. In combination with XPS, we propose a mechanism based on the synergistic effect between rim-based pentagonal rings and carbon radicals, which we believe can be exploited to produce other highly porous carbons. The CO2 capture capacity of the hyperporous carbon tested under dynamic CO2 capture conditions was ∼1.25 mmol g–1 versus 0.66 mmol g–1 of a conventionally activated carbon under similar conditions. The CO2 capture kinetics were extremely fast and reached 99% of the total capacity within 120 s. Lastly, supercapacitor electrodes deliver a high volumetric energy density of ∼60 W h L–1 and a volumetric power density of 1 kW L–1, which is the highest reported value for activated carbon.
通过超醇化和活化反应合成的超高表面积纳米多孔碳可提高二氧化碳容量和体积能量密度
我们报告了通过在模板基底上整合超醇与活化反应合成的碳吸附剂系列。这种材料设计产生的纳米多孔碳的 BET 面积为 4800 m2 g-1,总孔容积为 2.7 cm3 g-1,令人印象深刻。据我们所知,这个 BET 面积值是文献报道的最高值。电子自旋共振 (ESR) 测量确定了自由基的数量,以提供对超高比表面积碳形成机理的理解。结合 XPS,我们提出了一种基于边缘五角环和碳基之间协同效应的机制,我们相信可以利用这种机制生产出其他高多孔碳。在动态二氧化碳捕集条件下测试的超多孔碳的二氧化碳捕集能力为 1.25 mmol g-1,而传统活性碳在类似条件下的捕集能力为 0.66 mmol g-1。最后,超级电容器电极的体积能量密度高达 ∼60 W h L-1,体积功率密度为 1 kW L-1,这是活性炭的最高值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


