Plasmon-enhanced hydrogen production from photocatalytic methanol aqueous-phase reforming over novel nickel-tin intermetallic compounds photocatalyst
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Hydrogen production from the aqueous phase reforming (APR) of methanol is promising to reduce the global carbon dioxide emission, but it is still a great challenge for hydrogen production with high catalytic efficiency at low reaction temperature. Here we report that Al2O3 supported Ni3Sn1 intermetallic compounds nanoparticles as new class of plasmonic photocatalyst and enables low temperature (150–190 °C), efficient hydrogen production (277mol g−1 min−1) and low CO and CH4 selectivity ( 0.1 %) through APR with the help of light irradiation. The hydrogen production rate of Ni3Sn1/Al2O3 is 8 times higher than that of conventional noble metal 3 wt% Pt/Al2O3 catalyst under the same optimized reaction conditions. The exceptional photocatalytic performance for hydrogen production can be attribute to higher efficiency of “hot electron” transference into the reactant due to the higher energy level of “hot electron” and the lower energy level of metal-reactant adsorption bond induced by Sn doping in Ni3Sn1 under light irradiation, which not only improve the reactivity via enhanced methanol dissociation and the sequential water–gas shift (WGS) reaction, but also suppressed catalyst poisoning via accelerated CO2 desorption over the Ni sites.
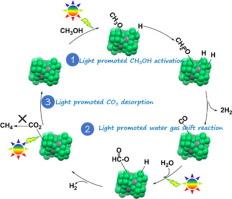
新型镍锡金属间化合物光催化剂光催化甲醇水相转化过程中的等离子体增强型制氢技术
甲醇水相重整(APR)制氢有望减少全球二氧化碳排放,但在低反应温度下实现高催化效率制氢仍是一项巨大挑战。在此,我们报告了 Al2O3 支持的 Ni3Sn1 金属间化合物纳米粒子作为新型等离子体光催化剂,在光照射的帮助下通过 APR 实现了低温(150-190 ℃)、高效制氢(277μμmol g-1 min-1)和低 CO 和 CH4 选择性(≤ 0.1 %)。在相同的优化反应条件下,Ni3Sn1/Al2O3 的制氢率是传统贵金属 3 wt% Pt/Al2O3 催化剂的 8 倍。这种优异的光催化制氢性能可归因于在光照射下,Ni3Sn1 中掺杂的 Sn 所诱导的更高能量级的 "热电子 "和更低能量级的金属-反应物吸附键使 "热电子 "转移到反应物中的效率更高,这不仅通过增强甲醇解离和水-气转移(WGS)顺序反应提高了反应活性,还通过加速 Ni 位点上的 CO2 解吸抑制了催化剂中毒。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


