Treatment of cosmetic wastewater containing N, N-dimethylformamide and high concentration of chloride salt by chemical precipitation and electrochemical method
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
High-strength wastewater containing elevated levels of chloride salt and N, N-dimethylformamide (DMF) solvent was collected from manufacturing of sunscreen cream (for UVA/UVB protection) at a cosmetic factory. In evaporation process, precipitates, formed due to the high chloride content (around 160 g/L), clog the pipeline, seriously reducing the treatment efficiency. This study aimed to develop a two-stage process integrating chemical precipitation and electrochemical oxidation to specifically remove the concentrated chloride salt and organic compounds (COD >100 g/L). Chloride ions were initially recovered as insoluble Friedel's salt using calcium hydroxide (Ca(OH)2) and sodium aluminate (NaAlO2) as the precipitants. The [Cl]/[Ca]/[Al] ratio and solution pH were optimized to obtain a pure crystallized phase of Ca2AlCl(OH)6•2H2O. Afterwards, the organic compound were treated by a Fered-Fenton with the addition of H2O2 and FeSO4 to degrade the remaining COD. The cost and energy consumption of this integrated processes were evaluated, compared to the conventional evaporation method.
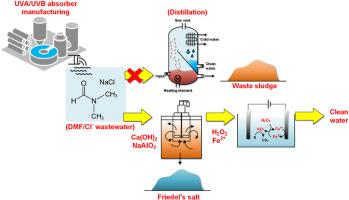
用化学沉淀和电化学方法处理含有 N,N-二甲基甲酰胺和高浓度氯盐的化妆品废水。
一家化妆品工厂在生产防晒霜(用于 UVA/UVB 防护)时收集了高强度废水,其中含有大量氯盐和 N, N-二甲基甲酰胺 (DMF) 溶剂。在蒸发过程中,由于氯化物含量高(约 160 g L-1)而形成的沉淀物堵塞了管道,严重降低了处理效率。本研究旨在开发一种集化学沉淀和电化学氧化于一体的两阶段工艺,专门去除高浓度氯盐和有机化合物(COD > 100 g L-1)。以氢氧化钙 (Ca(OH)2) 和铝酸钠 (NaAlO2) 作为沉淀剂,氯离子最初以不溶性弗里德尔盐的形式被回收。通过优化[Cl]/[Ca]/[Al]比率和溶液 pH 值,获得了 Ca2AlCl(OH)6-2H2O 的纯结晶相。之后,有机化合物通过加入 H2O2 和 FeSO4 的铁-芬顿法进行处理,以降解剩余的 COD。与传统的蒸发法相比,对这种综合工艺的成本和能耗进行了评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


