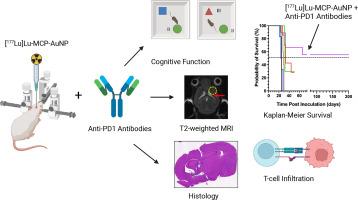
Convection-enhanced delivery of [177Lu]Lu-labeled gold nanoparticles combined with anti-PD1 checkpoint immunotherapy improves the survival of immunocompetent C57BL/6J mice with orthotopic GL261 murine glioma tumors
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
Abstract
Introduction
Our objective was to study convection enhanced delivery (CED) of 177Lu-labeled metal chelating polymer (MCP) conjugated to gold nanoparticles ([177Lu]Lu-MCP-AuNP) alone or combined with anti-PD1 immune checkpoint inhibition (ICI) for improving the survival of immunocompetent C57BL/6J mice with orthotopic GL261 murine glioma tumors.
Methods
C57BL/6J mice with GL261 tumors were treated with [177Lu]Lu-MCP-AuNP (0.8 or 2.7 MBq; 4 × 1011 AuNP) alone or combined with anti-PD1 antibodies (200 μg i.p. every 2 d × 3 doses). Control mice received normal saline, non-radioactive MCP-AuNP or anti-PD1 antibodies. Kaplan-Meier median survival was estimated. T-cell infiltration into the brain was probed by flow cytometry. Toxicity was assessed by monitoring body weight and cognitive function tests [Object Location Test (OLT) and Novel Object Recognition Test (NORT)] and T2-weighted MRI of the brain, overall health and ex vivo histopathological examination of the brain.
Results
Treatment with [177Lu]Lu-MCP-AuNP (0.8 MBq) significantly increased median survival compared to MCP-AuNP (29 vs. 25 d; P = 0.007) or normal saline-treated mice (24 d; P < 0.001). Combining [177Lu]Lu-MCP-AuNP (0.8 MBq) with anti-PD1 antibodies increased median survival to 32 d (P < 0.0001 vs. normal saline). Increasing the mean amount of [177Lu]Lu-MCP-AuNP to 2.7 MBq and combining with anti-PD1 antibodies extended survival to at least 218 d in 5/9 mice. Increased CD8+ cytotoxic T-cells and decreased CD4+ helper T-cells were found in the brain vs. normal saline-treated mice. No weight loss (>20 %) was observed for treated or control mice. There was no change in cognitive function in mice treated with [177Lu]Lu-MCP-AuNP (0.8 MBq) alone or combined with anti-PD1 antibodies assessed by the OLT or NORT. T2-weighted MRI in mice treated with 2.7 MBq [177Lu]Lu-MCP-AuNP combined with anti-PD1 antibodies revealed edema, gliosis and ex vacuo dilatation of the ventricle proximal to the site of infusion. Histopathological examination of the brain revealed dilatation of the ventricle and gliosis proximal to the site of infusion but no radiation necrosis. MRI and histological analysis did not reveal tumor in the brain of these mice. Mice treated with 2.7 MBq [177Lu]Lu-MCP-AuNP combined with anti-PD1 antibodies did not demonstrate overall deleterious health effects.
Conclusions
We conclude that CED of [177Lu]Lu-MCP-AuNP combined with anti-PD1 checkpoint immunotherapy improved the survival of immunocompetent C67BL/6J mice with GL261 glioma tumors in the brain. Higher administered amounts of [177Lu]Lu-MCP-AuNP (2.7 MBq vs. 0.8 MBq) were most effective and yielded long-term survival.
Advances in knowledge and implications for patient care
This study demonstrates that combining a locally-infused radiation nanomedicine, [177Lu]Lu-MCP-AuNP and anti-PD1 checkpoint immunotherapy improved the survival of mice with glioma tumors in the brain. In the future, this treatment may be useful to treat residual tumor at the surgical margins in patients with GBM to prevent local recurrence and improve survival.
对流增强型[177Lu]Lu标记金纳米粒子输送与抗PD1检查点免疫疗法相结合,提高了免疫功能正常的C57BL/6J小鼠正位GL261鼠胶质瘤肿瘤的存活率。
简介:我们的目的是研究177Lu标记的金属螯合聚合物(MCP)与金纳米颗粒([177Lu]Lu-MCP-AuNP)单独或与抗PD1免疫检查点抑制剂(ICI)联合的对流增强递送(CED),以改善免疫功能正常的C57BL/6J小鼠正位GL261鼠胶质瘤肿瘤的生存:方法:用[177Lu]Lu-MCP-AuNP(0.8 或 2.7 MBq;4 × 1011 AuNP)单独或与抗 PD1 抗体(200 μg i.p. 每 2 d × 3 次)联合治疗患有 GL261 肿瘤的 C57BL/6J 小鼠。对照组小鼠接受生理盐水、非放射性 MCP-AuNP 或抗 PD1 抗体治疗。估计卡普兰-梅耶尔中位生存率。流式细胞术检测了T细胞对大脑的浸润。毒性通过监测体重、认知功能测试[物体定位测试(OLT)和新物体识别测试(NORT)]、脑部T2加权核磁共振成像、总体健康状况和脑部体外组织病理学检查进行评估:177Lu]Lu-MCP-AuNP(0.8 MBq)与MCP-AuNP(29天 vs. 25天;P = 0.007)或正常生理盐水处理的小鼠(24天;P 177Lu]Lu-MCP-AuNP(0.8 MBq)与抗-PD1 抗体结合可将 5/9 只小鼠的中位生存期延长至 32 d(P 177Lu]Lu-MCP-AuNP 为 2.7 MBq,与抗-PD1 抗体结合可将 5/9 只小鼠的生存期延长至至少 218 d)。与正常生理盐水处理的小鼠相比,大脑中 CD8+细胞毒性 T 细胞增加,CD4+辅助性 T 细胞减少。治疗组和对照组小鼠的体重均未下降(>20%)。经 OLT 或 NORT 评估,单独或与抗 PD1 抗体联合使用 [177Lu]Lu-MCP-AuNP(0.8 MBq)治疗的小鼠认知功能没有变化。用 2.7 MBq [177Lu]Lu-MCP-AuNP(0.8 MBq)与抗-PD1 抗体联合治疗小鼠的 T2 加权核磁共振成像显示,输注部位近端脑室出现水肿、胶质增生和空泡扩张。脑组织病理学检查显示,输注部位近端脑室扩张和胶质增生,但没有放射性坏死。核磁共振成像和组织学分析未发现这些小鼠脑部有肿瘤。用2.7 MBq [177Lu]Lu-MCP-AuNP与抗PD1抗体联合治疗小鼠,总体上未显示出有害健康的影响:我们得出结论:[177Lu]Lu-MCP-AuNP的CED与抗PD1检查点免疫疗法相结合,提高了脑部患有GL261胶质瘤的免疫功能正常的C67BL/6J小鼠的生存率。给药量较高的[177Lu]Lu-MCP-AuNP(2.7 MBq对0.8 MBq)最有效,并能获得长期生存:这项研究表明,将局部注入的放射纳米药物[177Lu]Lu-MCP-AuNP与抗PD1检查点免疫疗法相结合,可提高脑胶质瘤小鼠的生存率。未来,这种治疗方法可用于治疗GBM患者手术边缘的残余肿瘤,以防止局部复发并提高生存率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


