Enzymatic nanomotors with chemotaxis for product-based cancer therapy
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The development of an intelligent nanomotor system holds great promise for enhancing the efficiency and effectiveness of antitumor therapy. Leveraging the overexpressed substances in the tumor microenvironment as propellants and chemotactic factors for enzyme-powered nanomotors represents a versatile and compelling approach. Herein, a plasma amine oxidase (PAO)-based chemotactic nanomotor system has been successfully developed, with the ability to enzymatically produce toxic acrolein and H2O2 from the upregulated polyamines (PAs) in the tumor microenvironment for active tumor therapy. Zwitterionic polymeric nanoparticles with superior biocompatibility are synthesized, followed by PAO modification via electrostatic interactions. As expected, the resulting nanomotor system exhibits positive chemotaxis toward PAs concentration gradient. Upon reaching the tumor region, our nanomotors, actuated by the tumor microenvironmental PAs, effectively enhance diffusion and enable deep penetration into the tumor site. This leads to the induction of tumor apoptosis and simultaneous inhibition of tumor invasion and migration by decomposing PAs into toxic products. By smartly utilizing the consumption of these local chemotactic factors and their enzymatic products, our nanomotor system provides a versatile and intelligent platform for active and enhanced tumor therapy.
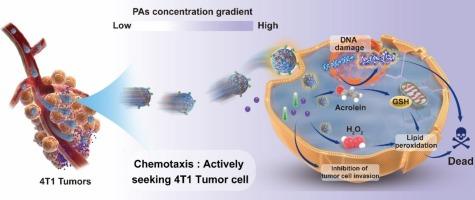
具有趋化性的酶纳米马达,用于基于产品的癌症治疗。
智能纳米电机系统的开发为提高抗肿瘤治疗的效率和效果带来了巨大希望。利用肿瘤微环境中的过表达物质作为酶动力纳米马达的推进剂和趋化因子,是一种多功能且引人注目的方法。在此,我们成功开发了一种基于血浆胺氧化酶(PAO)的化学趋化纳米马达系统,该系统能够利用肿瘤微环境中上调的多胺(PAs)酶促产生有毒的丙烯醛和 H2O2,从而达到主动治疗肿瘤的目的。首先合成了具有良好生物相容性的聚合纳米粒子,然后通过静电相互作用对 PAO 进行修饰。不出所料,由此产生的纳米马达系统对 PAs 浓度梯度表现出积极的趋化性。到达肿瘤区域后,我们的纳米马达在肿瘤微环境 PAs 的驱动下,有效地增强了扩散,并能深入肿瘤部位。通过将 PAs 分解成有毒产物,诱导肿瘤细胞凋亡,同时抑制肿瘤的侵袭和迁移。通过巧妙地利用这些局部趋化因子及其酶产物的消耗,我们的纳米电机系统为主动和强化肿瘤治疗提供了一个多功能的智能平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


