OAS1 induces endothelial dysfunction and promotes monocyte adhesion through the NFκB pathway in atherosclerosis
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cardiovascular disease is characterized by chronic inflammation and atherosclerosis (AS) is the pathological basis. Mitigating endothelial dysfunction and mononuclear cell adhesion is a crucial approach in impeding the initial advancement of AS. As an inflammation-immune regulation-related protein, 2′-5′-oligoadenylate synthetase 1 (OAS1) plays a critical role in inflammation, but its impact on endothelial dysfunction and mononuclear cell adhesion is not well understood. In this study, bioinformatic analysis revealed a significant enrichment of OAS1 in atherosclerotic plaques within human aortic sections. In addition, OAS1 was detected in atherosclerotic plaques within human aortic sections across various stages of development, with elevated expression observed in more advanced plaques. The expression of OAS1 exhibited a distinct temporal and concentration-dependent upregulation in response to lipopolysaccharide (LPS) stimulation. Notably, the deficiency of OAS1 markedly attenuated the elevation in reactive oxygen species (ROS) levels, nitric oxide (NO) concentrations, and monocyte adhesion induced by LPS. A positive correlation was observed between the levels of NFκBp65 and OAS1 in human plaques, and the deletion of OAS1 led to a down-regulation of P65 expression. Furthermore, the simultaneous knockdown of OAS1 and NFκBp65 resulted in a significant amelioration of endothelial dysfunction (including ROS, NO, and inflammation factors) and monocyte adhesion, suggesting a synergistic interaction between OAS1 and NFκBp65. These findings underscore the potential of OAS1 to modulate the extent of endothelial dysfunction and monocyte adhesion through its regulation of NFκBp65 thereby positioning it as a promising therapeutic target for the management of AS.
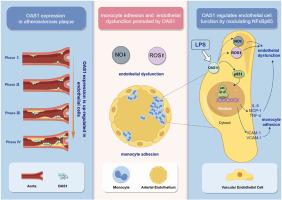
在动脉粥样硬化中,OAS1 通过 NFκB 通路诱导内皮功能障碍并促进单核细胞粘附。
心血管疾病的特点是慢性炎症,而动脉粥样硬化(AS)是其病理基础。缓解内皮功能障碍和单核细胞粘附是阻碍动脉粥样硬化初期发展的关键方法。作为一种炎症-免疫调节相关蛋白,2'-5'-醇化腺苷酸合成酶1(OAS1)在炎症中发挥着关键作用,但它对内皮功能障碍和单核细胞粘附的影响却不甚了解。在这项研究中,生物信息学分析显示,OAS1 在人体主动脉切片中的动脉粥样硬化斑块中明显富集。此外,在人体主动脉切片中不同发展阶段的动脉粥样硬化斑块中都检测到了 OAS1,在较晚期的斑块中观察到了更高的表达。在脂多糖(LPS)刺激下,OAS1的表达表现出明显的时间和浓度依赖性上调。值得注意的是,OAS1的缺乏明显减轻了LPS诱导的活性氧(ROS)水平、一氧化氮(NO)浓度和单核细胞粘附性的升高。在人类斑块中,NFκBp65和OAS1的水平呈正相关,而OAS1的缺失会导致P65表达的下调。此外,同时敲除 OAS1 和 NFκBp65 还能显著改善内皮功能障碍(包括 ROS、NO 和炎症因子)和单核细胞粘附,这表明 OAS1 和 NFκBp65 之间存在协同作用。这些发现强调了OAS1通过调节NFκBp65来调节内皮功能障碍和单核细胞粘附程度的潜力,从而将其定位为治疗强直性脊柱炎的一个有前景的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


