Galectin-1 Elicits a Tissue-Specific Anti-Inflammatory and Anti-Degradative Effect Upon LPS-Induced Response in an Ex Vivo Model of Human Fetal Membranes Modeling an Intraamniotic Inflammation
Abstract
Problem
Intrauterine infection is one of the most jeopardizing conditions associated with adverse outcomes, including preterm birth; however, multiple tolerance mechanisms operate at the maternal–fetal interface to avoid the rejection of the fetus. Among the factors that maintain the uterus as an immunoprivileged site, Galectin-1 (Gal-1), an immunomodulatory glycan-binding protein secreted by the maternal-fetal unit, is pivotal in promoting immune cell homeostasis. This work aimed to evaluate the role of Gal-1 during a lipopolysaccharide (LPS)-induced-inflammatory milieu.
Method of Study
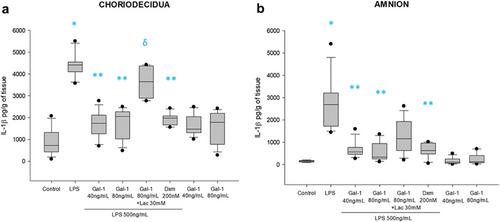
Using an ex vivo culture with two independent compartments, human fetal membranes at term were pretreated with 40 and 80 ng/mL of Gal-1, then to reproduce an intraamniotic inflammation, the fetal side of membranes was stimulated with 500 ng/mL of LPS for 24 h. The concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, monocyte chemoattractant protein (MCP1), macrophage inflammatory protein (MIP1) α, regulated upon activation normal T cell expressed and secreted (RANTES), and matrix metalloproteinase (MMP)-9 were measured in both amnion and choriodecidua compartments.
Results
In a tissue-specific fashion profile, pretreatment with the physiologic concentration of Gal-1 significantly diminished the LPS-dependent secretion of TNF-α, IL-1β, Il-6, MCP1, MIP1α, RANTES, and MMP-9.
Conclusion
Gal-1 elicits an anti-inflammatory effect on the human fetal membranes stimulated with LPS, which supports the hypothesis that Gal-1 is part of the immunomodulatory mechanisms intended to stop the harmful effect of inflammation of the maternal–fetal interface.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


