Dual-Targeted Assembled Nanodrugs for Near-Infrared Photothermal Immunotherapy of Triple-Negative Breast Cancer
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
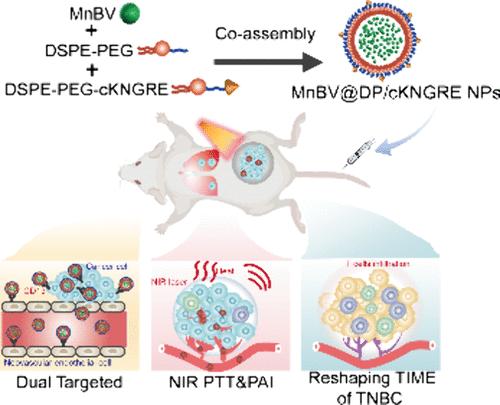
Triple-negative breast cancer (TNBC) is known for its poor prognosis and aggressive behavior, being highly prone to recurrence and metastasis, and currently has limited effective treatment options. Photothermal therapy (PTT) is an emerging, minimally invasive, low-drug-resistance, and precisely controllable therapeutic method for cancer treatment, offering hope to break through the bottleneck in TNBC therapy. The antitumor efficiency of PTT is predominantly contingent upon the performance of the photothermal drugs. Therefore, there is an urgent need to develop photothermal drugs that not only have excellent photothermal conversion efficiency but also possess strong tumor-targeting capabilities and good biosafety. Here, we have developed a tumor-targeted photothermal agent with near-infrared (NIR) absorption capability based on the strategy of biomolecular assembly, utilizing biliverdin manganese complexes (MnBV) and amphiphilic phospholipid–polymer conjugates (DSPE-PEG and DSPE-PEG-cKNGRE). This photothermal assembled drug exhibits a uniform size, good stability, and ideal photothermal conversion efficiency. In the 4T1 tumor-bearing mouse model of TNBC, it shows good tumor dual-targeting capabilities and a significant drug enrichment performance. While ablating the primary tumor, PTT further stimulates the maturation of dendritic cells (DCs), enhancing the infiltration of T lymphocytes into the spleen and tumor, thus reshaping the immune microenvironment of TNBC and thereby effectively inhibiting tumor metastasis and recurrence. The developed photothermal assembled drug provides an innovative candidate treatment paradigm for TNBC, offering the potential to advance precise, targeted, and safe therapy for highly invasive and aggressive malignancies.
用于三阴性乳腺癌近红外光热免疫疗法的双靶向组装纳米药物
三阴性乳腺癌(TNBC)以预后差和侵袭性强著称,极易复发和转移,目前有效的治疗方案有限。光热疗法(PTT)是一种新兴的微创、低耐药性、可精确控制的癌症治疗方法,为突破 TNBC 治疗瓶颈带来了希望。PTT 的抗肿瘤效率主要取决于光热药物的性能。因此,迫切需要开发出不仅具有优异的光热转换效率,而且具有较强的肿瘤靶向能力和良好的生物安全性的光热药物。在此,我们基于生物分子组装策略,利用双白藜芦醇锰复合物(MnBV)和两亲性磷脂-聚合物共轭物(DSPE-PEG 和 DSPE-PEG-cKNGRE),开发了一种具有近红外吸收能力的肿瘤靶向光热药物。这种光热组装药物大小均匀,稳定性好,光热转换效率理想。在 TNBC 的 4T1 肿瘤小鼠模型中,它表现出良好的肿瘤双靶向能力和显著的药物富集性能。在消融原发肿瘤的同时,PTT还能进一步刺激树突状细胞(DCs)成熟,增强T淋巴细胞对脾脏和肿瘤的浸润,从而重塑TNBC的免疫微环境,有效抑制肿瘤转移和复发。所开发的光热装配药物为 TNBC 提供了一种创新的候选治疗范例,为推进高侵袭性和侵袭性恶性肿瘤的精确、靶向和安全治疗提供了可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


