Elucidating the Discharge Behavior of Aqueous Zinc Sulfur Batteries in the Presence of Molybdenum(IV) Chalcogenide Catalyst: The Criticality of Interfacial Electrochemistry
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The aqueous zinc–sulfur battery holds promise for significant capacity and energy density with low cost and safe operation based on environmentally benign materials. However, it suffers from the sluggish kinetics of the conversion reaction. Here, we highlight the efficacy of molybdenum(IV) sulfide (MoS2) to reduce the overpotential of S-ZnS conversion in aqueous electrolytes and study the discharge products formed at the solid–solid and solid–liquid interfaces using experimental and theoretical approaches. Specifically, the MoS2-catalyzed electrochemical conversion reaction is characterized via ex situ X-ray diffraction (XRD), transmission electron microscopy (TEM) with energy dispersive spectroscopy (EDS), Raman spectroscopy, synchrotron-based Mo K-edge X-ray absorption spectroscopy (XAS), and in situ synchrotron-based X-ray computed tomography (XCT). Additionally, operando synchrotron-based S K-edge XAS and X-ray fluorescence (XRF) maps are collected to determine the spatial evolution of sulfur-based species at the electrode–electrolyte interface. Coupling the operando S K-edge XAS data with the simulated spectra and fitting the data suggested a possible ZnS2 intermediate phase.
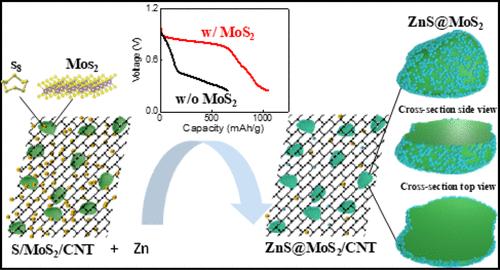
阐明存在卤化钼(IV)催化剂的水性锌硫电池的放电行为:界面电化学的临界性
锌硫水溶液电池采用对环境无害的材料,具有容量大、能量密度高、成本低、运行安全等优点。然而,它却受到转化反应动力学缓慢的影响。在此,我们重点介绍了硫化钼(IV)(MoS2)在水性电解质中降低 S-ZnS 转换过电位的功效,并利用实验和理论方法研究了在固-固和固-液界面形成的放电产物。具体来说,MoS2 催化的电化学转化反应是通过原位 X 射线衍射 (XRD)、透射电子显微镜 (TEM) 与能量色散光谱 (EDS)、拉曼光谱、同步辐射 Mo K-edge X 射线吸收光谱 (XAS) 和原位同步辐射 X 射线计算机断层扫描 (XCT) 来表征的。此外,还收集了基于同步辐射的 S K-edge XAS 和 X 射线荧光 (XRF) 图谱,以确定电极-电解质界面上硫基物种的空间演化。将操作面 S K-edge XAS 数据与模拟光谱耦合并对数据进行拟合后,发现可能存在 ZnS2 中间相。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


