In Situ Phase Transformation-Enabled Metal–Organic Frameworks for Efficient CO2 Electroreduction to Multicarbon Products in Strong Acidic Media
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electrochemical CO2 reduction reaction (CO2RR) has been acknowledged as a promising strategy to relieve carbon emissions by converting CO2 to essential chemicals. Despite significant progresses that have been made in neutral and alkaline media, the implementation of CO2RR in acidic conditions remains challenging due to the harsh conditions, especially in producing high-value multicarbon products. Here, we report that Cu-btca (btca = benzotriazole-5-carboxylic acid) metal–organic framework (MOF) nanostructures can act as a stable catalyst for the CO2RR in an acidic environment. The Cu-btca MOF undergoes phase transformation and morphology evolution during electrolysis, forming a stable porous Cu-btca MOF network. The resultant MOF network exhibits excellent selectivity toward ethylene and multicarbon products with Faradaic efficiencies of 51.2% and 81.9%, respectively, in a strong acidic electrolyte with a flow cell at 300 mA/cm2. Mechanism studies uncover that the Cu-btca MOF network can limit the proton reduction to suppress hydrogen evolution and maintain high local *CO concentration to promote CO2RR. Theoretical calculations suggest that two adjacent Cu sites in the Cu-btca MOF provide a favorable microenvironment for carbon–carbon coupling, facilitating the multicarbon production. This work reveals that rational structure control of MOFs can enable highly selective and efficient CO2 electroreduction to multicarbon products in strong acidic conditions toward practical applications.
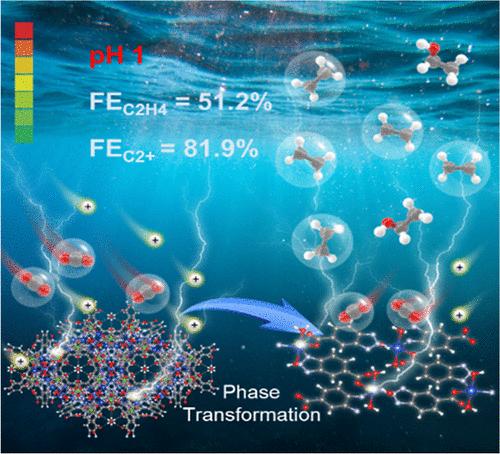
在强酸性介质中通过原位相变实现金属有机框架,将二氧化碳高效电还原为多碳产品
电化学二氧化碳还原反应(CO2RR)被认为是通过将二氧化碳转化为必需化学品来减少碳排放的一种有前途的策略。尽管在中性和碱性介质中已经取得了重大进展,但由于条件苛刻,特别是在生产高价值多碳产品时,在酸性条件下实施 CO2RR 仍具有挑战性。在此,我们报告了 Cu-btca(btca = 苯并三唑-5-羧酸)金属有机框架(MOF)纳米结构可在酸性环境中作为稳定的 CO2RR 催化剂。Cu-btca MOF 在电解过程中会发生相变和形态演变,形成稳定的多孔 Cu-btca MOF 网络。由此形成的 MOF 网络对乙烯和多碳产品具有极佳的选择性,在强酸性电解质和 300 mA/cm2 的流动池中,法拉第效率分别为 51.2% 和 81.9%。机理研究发现,Cu-btca MOF 网络可以限制质子还原,从而抑制氢气进化,并保持较高的局部 *CO 浓度,以促进 CO2RR。理论计算表明,Cu-btca MOF 中相邻的两个 Cu 位点为碳-碳耦合提供了有利的微环境,促进了多碳生成。这项工作揭示了合理控制 MOF 的结构可以在强酸条件下实现高选择性和高效的 CO2 电还原为多碳产物,从而实现实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


