Frizzled5 controls murine intestinal epithelial cell plasticity through organization of chromatin accessibility
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
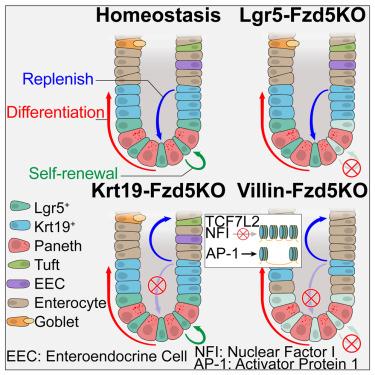
The homeostasis of the intestinal epithelium relies on intricate yet insufficiently understood mechanisms of intestinal epithelial plasticity. Here, we elucidate the pivotal role of Frizzled5 (Fzd5), a Wnt pathway receptor, as a determinant of murine intestinal epithelial cell fate. Deletion of Fzd5 in Lgr5+ intestinal stem cells (ISCs) impairs their self-renewal, whereas its deletion in Krt19+ cells disrupts lineage generation, without affecting crypt integrity in either case. However, a broader deletion of Fzd5 across the epithelium leads to substantial crypt deterioration. Integrated analysis of single-cell RNA sequencing (scRNA-seq) and single-cell ATAC-seq (scATAC-seq) identifies that Fzd5 governs chromatin accessibility, orchestrating the regulation of stem- and lineage-related gene expression mainly in ISCs and progenitor cells. In summary, our findings provide insights into the regulatory role of Fzd5 in governing intestinal epithelial plasticity.
Frizzled5通过组织染色质可及性控制小鼠肠上皮细胞的可塑性
肠上皮细胞的稳态依赖于错综复杂的肠上皮细胞可塑性机制,但人们对这种机制的了解还不够。在这里,我们阐明了Wnt通路受体Frizzled5(Fzd5)作为小鼠肠上皮细胞命运决定因素的关键作用。在Lgr5+肠干细胞(ISCs)中缺失Fzd5会影响它们的自我更新,而在Krt19+细胞中缺失Fzd5则会破坏细胞系的生成,但两种情况都不会影响隐窝的完整性。然而,在上皮细胞中更广泛地缺失 Fzd5 会导致隐窝严重恶化。单细胞RNA测序(scRNA-seq)和单细胞ATAC-seq(scATAC-seq)的综合分析表明,Fzd5控制染色质的可及性,主要在ISC和祖细胞中协调调控干系和系谱相关基因的表达。总之,我们的研究结果为了解 Fzd5 在肠上皮可塑性中的调控作用提供了深入的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


