FZD5 controls intestinal crypt homeostasis and colonic Wnt surrogate agonist response
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The rapidly regenerating intestinal epithelium requires crypt intestinal stem cells (ISCs). Wnt/β-catenin signaling maintains crypt homeostasis and Lgr5+ ISCs, and WNT ligands bind Frizzled receptors (FZD1–10). Identifying specific FZD(s) essential for intestinal homeostasis has been elusive; however, bioengineered antagonists blocking Wnt binding to FZD5 and FZD8 deplete the gut epithelium in vivo, highlighting potential roles. Here, an epithelial-specific Fzd5 knockout (KO) elicited lethal pan-intestinal crypt and villus loss, whereas an Lgr5+ ISC-specific Fzd5 KO depleted Lgr5+ ISCs via premature differentiation and repressed Wnt target genes. Fzd5-null phenotypes were rescued by constitutive β-catenin activation in vivo and in both mouse and human enteroids. KO of Fzd5, not Fzd8, in enteroids ablated responsiveness to dual-specificity FZD5/FZD8-selective Wnt surrogate agonists, which ameliorated DSS-induced colitis in wild-type and Fzd8 KO mice. Overall, FZD5 is a dominant and essential regulator of crypt homeostasis, Lgr5+ ISCs, and intestinal response to Wnt surrogate agonists, with implications for therapeutic mucosal repair.
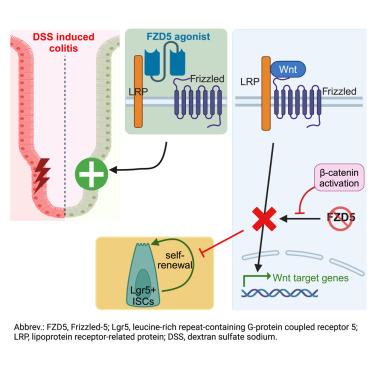
FZD5控制肠隐窝稳态和结肠Wnt替代激动剂反应
快速再生的肠上皮需要隐窝肠干细胞(ISC)。Wnt/β-catenin信号维持隐窝稳态和Lgr5+ ISC,WNT配体与Frizzled受体(FZD1-10)结合。然而,阻断 Wnt 与 FZD5 和 FZD8 结合的生物工程拮抗剂会使体内肠道上皮细胞衰竭,从而突显出其潜在的作用。在这里,上皮特异性Fzd5基因敲除(KO)引起了致命的泛肠道隐窝和绒毛缺失,而Lgr5+ ISC特异性Fzd5基因敲除则通过过早分化和抑制Wnt靶基因耗竭了Lgr5+ ISC。Fzd5缺失的表型在体内以及小鼠和人类肠道中都能通过组成型β-catenin激活得到挽救。在肠道中 KO Fzd5(而非 Fzd8)会减弱对 FZD5/FZD8 选择性 Wnt 替代激动剂的反应,从而改善野生型和 Fzd8 KO 小鼠 DSS 诱导的结肠炎。总之,FZD5是隐窝稳态、Lgr5+ ISC和肠道对Wnt替代激动剂反应的主要和基本调节因子,对治疗性粘膜修复具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


