Subnano Polyhydroxylated C60 and Co-oxo Clusters Enable Accelerated Electron Kinetics for CO2 Photoreduction in Pure Water
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Accelerating the electron kinetics is critical for enhancing the photocatalytic performance of CO2 conversion. Herein, polyhydroxylated C60 (C60OH, ∼1.8 nm) was molecularly decorated on boron-doped carbon nitride (BCN) nanosheets via a hydrogen-bonding assembly process. Subsequently, subnano Co-oxo clusters (∼0.4 nm) were precisely deposited on the BCN counterpart using a photohole-induced approach. The optimized nanocomposite photocatalyst, featuring spatially separated dual subnano modifiers, exhibits a 10-fold CO2 conversion rate of that for BCN in pure water and nearly 100% selectivity toward CO by completely inhibiting H2 evolution. Notably, the apparent quantum yield reaches 3.51% (405 nm), surpassing that of representative cocatalyst-involved (boron-doped) carbon nitride-based photocatalysts under similar conditions. Femtosecond-transient absorption spectra reveal that C60OH and Co-oxo clusters can rapidly extract electrons and holes, respectively, with balanced transfer rates. Moreover, the hydroxyl groups of C60OH can serve as CO2 adsorption and catalytic sites, whereas Co-oxo clusters are capable of catalyzing water oxidation. The synergy between dual subnano modifiers effectively improves the electron kinetics, resulting in an electron transfer efficiency of 41.1% determined by in situ microsecond-transient absorption spectroscopy. This work provides a rational design strategy for developing advanced photocatalysts by modulating electron kinetics.
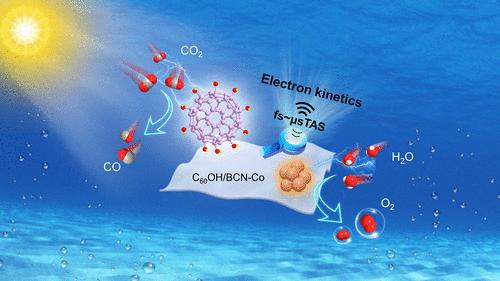
亚纳米级多羟基 C60 和共氧团簇可加速纯水中 CO2 光还原的电子动力学过程
加速电子动力学对于提高二氧化碳转化的光催化性能至关重要。在此,通过氢键组装工艺将多羟基 C60(C60OH,∼1.8 nm)分子装饰在掺硼氮化碳(BCN)纳米片上。随后,利用光孔诱导方法将亚纳米 Co-oxo 簇(∼0.4 nm)精确沉积在氮化硼纳米片上。优化后的纳米复合光催化剂具有空间分离的双重亚纳米改性剂,在纯水中的二氧化碳转化率是 BCN 的 10 倍,并且通过完全抑制 H2 的演化,对 CO 的选择性接近 100%。值得注意的是,其表观量子产率达到 3.51%(405 纳米),超过了类似条件下具有代表性的共催化剂参与型(掺硼)氮化碳基光催化剂。飞秒瞬态吸收光谱显示,C60OH 和 Co-oxo 团簇可分别快速萃取电子和空穴,且传输速率均衡。此外,C60OH 的羟基可以作为二氧化碳的吸附和催化位点,而 Co-oxo 团簇则能够催化水的氧化。双重亚纳米改性剂的协同作用有效地改善了电子动力学,通过原位微秒瞬态吸收光谱测定,电子转移效率高达 41.1%。这项工作为通过调节电子动力学开发先进光催化剂提供了合理的设计策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


