Histological severity, clinical outcomes and impact of antiviral treatment in indeterminate phase of chronic hepatitis B: a systematic review and meta-analysis
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Current international guidelines recommend close monitoring and evaluation of patients with chronic hepatitis B (CHB) in the indeterminate phase, and treatment of patients at high risk of adverse outcomes. Clinical outcomes and the effect of antiviral therapy on the indeterminate phase remain unclear. We performed a systematic review and meta-analysis to study the incidence of adverse clinical outcomes including hepatocellular carcinoma (HCC), cirrhosis, and hepatic decompensation, and the effect of antiviral therapy, in the indeterminate phase.Methods
Two investigators independently searched Embase, MEDLINE, Web of Science and China National Knowledge Infrastructure from 1/1/2007 to 31/12/2023. Three investigators independently assessed study eligibility and quality. We included cohort studies and randomised controlled trials (RCTs) allowing calculation of the incidence rate of adverse clinical outcomes, and cross-sectional studies that reported the prevalence of moderate-to-severe inflammation and different degrees of fibrosis. Incidence rates and prevalence were pooled using generalised linear mixed-effects models and random-effects models, respectively.Results
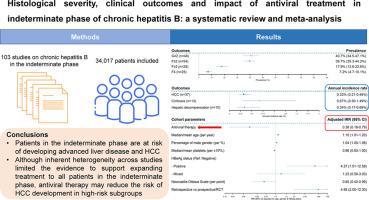
One hundred and three studies (70 case-control studies [18,739 patients], 32 cohort studies [15,118 patients], 1 RCT [160 patients]) were included. The annual incidence rate of HCC in patients in the indeterminate phase was 0.32% (95% CI 0.21-0.48%, I2=85.7%), and those of cirrhosis and hepatic decompensation were 0.67% (95% CI 0.30-1.49%, I2=94.3%) and 0.34% (95% CI 0.17-0.69%, I2=51.8%), respectively. The pooled prevalence of moderate-to-severe liver inflammation, significant fibrosis, advanced fibrosis, and cirrhosis was 40.7, 39.7%, 17.9%, and 7.2%, respectively. Use of antiviral therapy was associated with a lower risk of HCC in patients in the indeterminate phase (adjusted incidence rate ratio 0.38, 95% CI 0.18-0.79, p=0.009).Conclusions
Patients in the indeterminate phase are at risk of developing advanced liver disease and HCC. Although inherent heterogeneity across studies limited the evidence to support expanding treatment to all patients in the indeterminate phase, antiviral therapy may reduce the risk of HCC development in high-risk subgroups.IMPACT AND IMPLICATIONS
Current international guidelines recommend close monitoring and evaluation of patients with chronic hepatitis B (CHB) in the indeterminate phase, and not all of these patients are indicated for antiviral treatment. Based on the systematic review and meta-analysis with significant heterogeneity across studies, patients in the indeterminate phase are at risk of developing hepatocellular carcinoma (HCC), cirrhosis, and hepatic decompensation. Meta-regression findings on platelet count, positive HBeAg, and age highlighted the importance of liver fibrosis assessment, accurate phase classification, and timely detection of phase transition to identify antiviral treatment indications, supporting current guideline recommendations. Antiviral treatment may reduce risk of HCC in the high-risk subgroups of patients in the indeterminate phase.PROSPERO registration number
CRD42024537095.
慢性乙型肝炎不确定期的组织学严重程度、临床结果和抗病毒治疗的影响:系统回顾和荟萃分析
背景& 目的目前的国际指南建议对处于不确定期的慢性乙型肝炎(CHB)患者进行密切监测和评估,并对不良后果风险高的患者进行治疗。临床结果和抗病毒治疗对不确定期的影响仍不明确。我们进行了一项系统性回顾和荟萃分析,以研究未定阶段不良临床结局(包括肝细胞癌(HCC)、肝硬化和肝功能失代偿)的发生率以及抗病毒治疗的效果。方法:两位研究者从 2007 年 1 月 1 日至 2023 年 12 月 31 日独立检索了 Embase、MEDLINE、Web of Science 和中国国家知识基础设施。三名研究人员独立评估了研究的资格和质量。我们纳入了可计算不良临床结果发生率的队列研究和随机对照试验(RCT),以及报告中重度炎症和不同程度纤维化发生率的横断面研究。采用广义线性混合效应模型和随机效应模型分别对发病率和患病率进行了汇总。结果 共纳入 103 项研究(70 项病例对照研究 [18,739 名患者]、32 项队列研究 [15,118 名患者]、1 项 RCT [160 名患者])。处于不确定期患者的 HCC 年发病率为 0.32%(95% CI 0.21-0.48%,I2=85.7%),肝硬化和肝功能失代偿的年发病率分别为 0.67%(95% CI 0.30-1.49%,I2=94.3%)和 0.34%(95% CI 0.17-0.69%,I2=51.8%)。中重度肝脏炎症、显著肝纤维化、晚期肝纤维化和肝硬化的汇总患病率分别为 40.7%、39.7%、17.9% 和 7.2%。使用抗病毒治疗与处于不确定期的患者较低的 HCC 风险相关(调整后发病率比为 0.38,95% CI 为 0.18-0.79,P=0.009)。尽管不同研究之间固有的异质性限制了将治疗范围扩大到所有处于不确定期的患者的证据,但抗病毒治疗可降低高风险亚组患者发展为 HCC 的风险。影响和启示目前的国际指南建议对处于不确定期的慢性乙型肝炎(CHB)患者进行密切监测和评估,但并非所有这些患者都适合接受抗病毒治疗。根据系统综述和荟萃分析(各研究间存在显著异质性),处于不确定期的患者有发展为肝细胞癌(HCC)、肝硬化和肝功能失代偿的风险。关于血小板计数、HBeAg阳性和年龄的元回归结果突出了肝纤维化评估、准确的分期和及时发现分期转变对确定抗病毒治疗适应症的重要性,这也支持了目前的指南建议。抗病毒治疗可降低处于不确定期的高风险亚组患者发生 HCC 的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


