Real-world clinical data-driven modelling on the initiation time of antiviral prophylaxis among pregnant women with chronic hepatitis B infection
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
The risk of mother-to-child transmission (MTCT) for pregnant women with chronic hepatitis B (CHB) infection still exists, especially for those with high HBV DNA level. The guidelines for initiating prophylaxis for pregnant women with CHB vary across countries. We aimed to explore the latest prophylaxis initiation time for these women.Methods
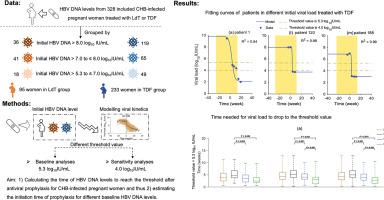
We collected the real-world clinical data of 328 pregnant women with CHB infection aged 20-49 treated by telbivudine (LdT) or tenofovir disoproxil fumarate (TDF) from July 2010 to December 2020 in China. A mathematical model was developed to describe the viral kinetics of HBV after prophylaxis. We calculated the time required to reduce viral load below the threshold value of 5.3 log10 IU/mL. We derived the prophylaxis initiation time by subtracting the required time to threshold from the childbirth gestational week.Results
The median time for 328 women to reduce HBV DNA levels below the threshold of 5.3 log10 IU/mL was 4.2 (range: 0.2-12.8) weeks, corresponding to prophylaxis initiation time of no later than 35.1 (25.2-41.4) weeks. Specifically, for women with viral loads > 8.0 log10 IU/mL, prophylaxis should be initiated before 33.9 (25.2-39.5) weeks, and particularly before the lower bound of 25.2 weeks, to maximize clinical safety. For women with viral load >7.0 to 8.0 log10 IU/mL, prophylaxis should be initiated before 35.5 (28.6-39.8) weeks, and for women with viral load >5.3 to 7.0 log10 IU/mL, prophylaxis should be initiated before 36.2 (28.3-41.4) weeks.Conclusion
Pregnant women with HBV DNA levels >5.3 to 8.0 log10 IU/mL can initiate prophylaxis before 28 gestational weeks. However, women with HBV DNA >8.0 log10 IU/mL could consider initiating prophylaxis before 25 weeks.Impact and implications
This study investigates how long it takes to decrease maternal viral load below a threshold·(5.3 log10 IU/mL) after receiving antiviral prophylaxis in pregnant women with different HBV DNA levels based on real-world clinical data and mathematical modelling, which provides quantitative evidence on the initiation time of antiviral prophylaxis. The results show that pregnant women with CHB infection at high HBV DNA levels (>8 log10 IU/mL) should initiate antiviral prophylaxis earlier to decrease the risk of mother-to-child transmission of HBV. Physicians can determine when to begin antiviral prophylaxis for those women according to their maternal HBV DNA levels. Our findings justify the initiation time of antiviral prophylaxis recommended by the Chinese guidelines and will offer new insights for other international guidelines.
关于慢性乙型肝炎感染孕妇开始抗病毒预防治疗时间的真实世界临床数据驱动模型
背景& 目的感染慢性乙型肝炎(CHB)的孕妇仍存在母婴传播(MTCT)的风险,尤其是那些 HBV DNA 水平较高的孕妇。各国为感染慢性乙型肝炎的孕妇制定的预防指南各不相同。我们收集了 2010 年 7 月至 2020 年 12 月期间中国 328 名 20-49 岁 CHB 感染孕妇接受替比夫定(LdT)或富马酸替诺福韦二吡呋酯(TDF)治疗的真实临床数据。我们建立了一个数学模型来描述预防治疗后 HBV 的病毒动力学。我们计算了将病毒载量降至 5.3 log10 IU/mL 临界值以下所需的时间。结果 328 名妇女将 HBV DNA 水平降至 5.3 log10 IU/mL 临界值以下所需的中位时间为 4.2 周(范围:0.2-12.8),与此相对应,开始预防的时间不晚于 35.1 周(25.2-41.4)。具体来说,对于病毒载量为8.0 log10 IU/mL的女性,应在33.9(25.2-39.5)周之前开始预防,尤其是在25.2周的下限之前,以最大限度地提高临床安全性。对于病毒载量为>7.0至[数学处理错误]≤≤8.0 log10 IU/mL的女性,应在35.5(28.6-39.8)周之前开始预防;对于病毒载量为>5.3至[数学处理错误]≤≤7.结论HBV DNA水平为>5.3至[数学处理错误]≤≤8.0 log10 IU/mL的孕妇可在孕28周前开始预防。影响和意义本研究基于真实世界的临床数据和数学模型,调查了不同 HBV DNA 水平的孕妇在接受抗病毒预防治疗后,母体病毒载量降低到阈值(5.3 log10 IU/mL)以下所需的时间,为抗病毒预防治疗的启动时间提供了定量证据。结果显示,HBV DNA 水平较高(>8 log10 IU/mL)的 CHB 感染孕妇应尽早开始抗病毒预防,以降低 HBV 母婴传播的风险。医生可根据产妇的 HBV DNA 水平决定何时开始对这些妇女进行抗病毒预防。我们的研究结果证明了中国指南所建议的抗病毒预防的开始时间是合理的,并将为其他国际指南提供新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


