Performance of xMg3Al1-LDH@ZIF-8 in High Efficiency Electrocatalytic Reduction of CO2 to CO
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This study involves the preparation of the precursor of magnesium aluminum layered double hydroxide (Mg3Al1-LDH) through a hydrothermal synthesis method. Subsequently, altering the loading amount of the precursor to synthesize a series of nanomaterials (xMg3Al1-LDH@ZIF-8, x = 0.2, 0.5, and 0.8) composite with zeolitic imidazolate framework-8 (ZIF-8). The investigation delves into the electrocatalytic performance of the material in the electrochemical reduction of CO2 (CO2RR) for the production of CO. The electrocatalyst is subjected to analysis through various techniques such as XRD, XPS, Raman, FTIR, SEM, EDS, TEM, BET, etc., to examine the elemental composition, microscopic morphology, and surface area with pore size. The electrochemical performance of the materials is tested and analyzed using an electrochemical workstation and gas chromatograph. The research findings reveal that the electrocatalyst with a loading amount of 0.5 g, denoted as 0.5Mg3Al1-LDH@ZIF-8, exhibits a well-defined rhombic dodecahedral morphology with a surface-attached layered structure. This structure, characterized by relatively strong interactions, provides abundant active sites for the reaction, consequently demonstrating superior electrochemical performance. The Faradaic efficiency (CO FE) for CO2RR to produce CO reaches a maximum of 88.08% at −1.5 V vs. RHE. Maintaining a constant applied voltage at −1.4 V vs. RHE ensures stability for up to 4 h.
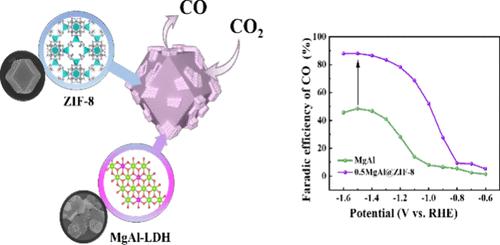
xMg3Al1-LDH@ZIF-8 在高效电催化还原 CO2 到 CO 中的性能
本研究通过水热合成法制备了镁铝层状双氢氧化物(Mg3Al1-LDH)的前驱体。随后,通过改变前驱体的负载量,合成了一系列与沸石咪唑酸框架-8(ZIF-8)复合的纳米材料(xMg3Al1-LDH@ZIF-8,x = 0.2、0.5 和 0.8)。研究深入探讨了该材料在电化学还原 CO2(CO2RR)以生产 CO 的过程中的电催化性能。通过 XRD、XPS、拉曼、傅立叶变换红外光谱、扫描电镜、EDS、TEM、BET 等多种技术对电催化剂进行分析,研究其元素组成、微观形貌、表面积和孔径。使用电化学工作站和气相色谱仪对材料的电化学性能进行了测试和分析。研究结果表明,负载量为 0.5g 的电催化剂(命名为 0.5Mg3Al1-LDH@ZIF-8)呈现出清晰的菱形十二面体形态,表面附着有层状结构。这种结构的特点是相互作用相对较强,为反应提供了丰富的活性位点,从而表现出卓越的电化学性能。CO2RR 生成 CO 的法拉第效率(CO FE)在-1.5 V 对 RHE 时达到最大值 88.08%。在-1.4 V(相对于 RHE)电压下保持恒定的施加电压可确保长达 4 小时的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


