The neonatal Fc receptor is a cellular receptor for human astrovirus
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
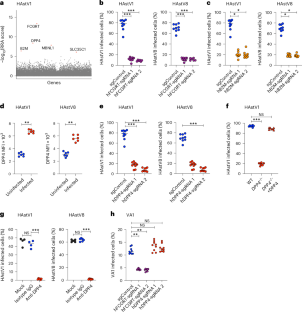
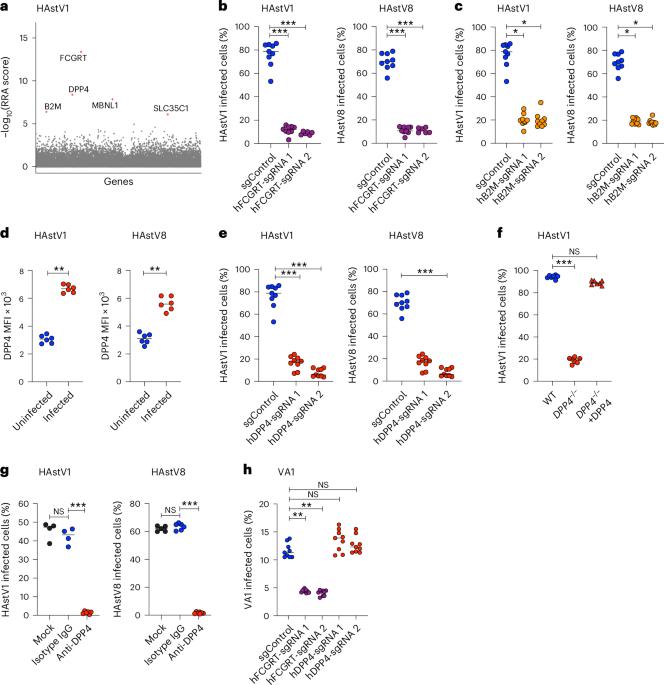
Human astroviruses (HAstV) are major causes of gastroenteritis, especially in children, and there are no vaccines or antivirals currently available. Little is known about host factors required for their cellular entry. Here we utilized complementary CRISPR-Cas9-based knockout and activation screens to identify neonatal Fc receptor (FcRn) and dipeptidyl-peptidase IV (DPP4) as entry factors for HAstV infection in vitro. Disruption of FcRn or DPP4 reduced HAstV infection in permissive cells and, reciprocally, overexpression of these factors in non-permissive cells was sufficient to promote infection. We observed direct binding of FcRn, but not DPP4, with HAstV virions and the purified spike protein. This suggests that FcRn is a receptor for HAstVs while DPP4 is a cofactor for entry. Inhibitors for DPP4 and FcRn currently in clinical use prevented HAstV infection in cell lines and human enteroids. Our results reveal mechanisms of HAstV entry as well as druggable targets to limit HAstV infection. A CRISPR-based screen identifies neonatal Fc receptor (FcRn) and dipeptidyl-peptidase IV (DPP4) as entry factors for human astrovirus, and targeting them using available therapies effectively prevents infection in human enteroid cultures.
新生儿 Fc 受体是人类星状病毒的细胞受体
人类星状病毒(HAstV)是导致肠胃炎,尤其是儿童肠胃炎的主要原因,目前还没有疫苗或抗病毒药物。人们对其进入细胞所需的宿主因子知之甚少。在这里,我们利用基于 CRISPR-Cas9 的互补性基因敲除和激活筛选,确定了新生儿 Fc 受体(FcRn)和二肽基肽酶 IV(DPP4)作为体外感染 HAstV 的进入因子。FcRn或DPP4的破坏会减少HAstV在允许细胞中的感染,反之,在非允许细胞中过表达这些因子足以促进感染。我们观察到 FcRn(而非 DPP4)与 HAstV 病毒和纯化的尖峰蛋白直接结合。这表明 FcRn 是 HAstV 的受体,而 DPP4 是进入的辅助因子。目前临床上使用的 DPP4 和 FcRn 抑制剂可阻止 HAstV 在细胞系和人体肠道中的感染。我们的研究结果揭示了 HAstV 的进入机制以及限制 HAstV 感染的药物靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


