ABA-activated low-nanomolar Ca2+–CPK signalling controls root cap cycle plasticity and stress adaptation
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
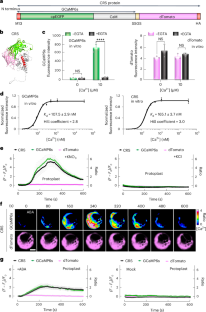
Abscisic acid (ABA) regulates plant stress adaptation, growth and reproduction. Despite extensive ABA–Ca2+ signalling links, imaging ABA-induced increases in Ca2+ concentration has been challenging, except in guard cells. Here we visualize ABA-triggered [Ca2+] dynamics in diverse organs and cell types of Arabidopsis thaliana using a genetically encoded Ca2+ ratiometric sensor with a low-nanomolar Ca2+-binding affinity and a large dynamic range. The subcellular-targeted Ca2+ ratiometric sensor reveals time-resolved and unique spatiotemporal Ca2+ signatures from the initial plasma-membrane nanodomain, to cytosol, to nuclear oscillation. Via receptors and sucrose-non-fermenting1-related protein kinases (SnRK2.2/2.3/2.6), ABA activates low-nanomolar Ca2+ transient and Ca2+-sensor protein kinase (CPK10/30/32) signalling in the root cap cycle from stem cells to cell detachment. Surprisingly, unlike the prevailing NaCl-stimulated micromolar Ca2+ spike, salt stress induces a low-nanomolar Ca2+ transient through ABA signalling, repressing key transcription factors that dictate cell fate and enzymes that are crucial to root cap maturation and slough. Our findings uncover ABA–Ca2+–CPK signalling that modulates root cap cycle plasticity in adaptation to adverse environments. This study reveals ABA-triggered low-nanomolar Ca2+ dynamics in diverse plant organs and cell types using an ultrasensitive Ca2+ biosensor. Spatiotemporal Ca2+ dynamics modulate the root cap cycle in adaptation to stress through ABA–Ca2+–CPK signalling.

ABA激活的低纳摩尔Ca2+-CPK信号控制根帽周期可塑性和胁迫适应性
脱落酸(ABA)调节植物的胁迫适应、生长和繁殖。尽管 ABA 与 Ca2+ 信号有广泛的联系,但除了在保卫细胞中,对 ABA 诱导的 Ca2+ 浓度增加进行成像一直是一项挑战。在这里,我们利用一种基因编码的 Ca2+ 比率传感器(具有低纳摩尔 Ca2+ 结合亲和力和较大的动态范围)对拟南芥不同器官和细胞类型中 ABA 触发的[Ca2+]动态进行了可视化。这种亚细胞靶向 Ca2+ 比率测定传感器揭示了从最初的质膜纳米域到细胞质再到核振荡的时间分辨和独特的时空 Ca2+ 特征。通过受体和蔗糖-非发酵1相关蛋白激酶(SnRK2.2/2.3/2.6),ABA激活了从干细胞到细胞脱落的根帽周期中的低纳摩尔Ca2+瞬时和Ca2+感应蛋白激酶(CPK10/30/32)信号。令人惊讶的是,与普遍的 NaCl 刺激微摩尔 Ca2+ 峰值不同,盐胁迫通过 ABA 信号诱导低纳摩尔 Ca2+ 瞬态,抑制决定细胞命运的关键转录因子以及对根帽成熟和脱落至关重要的酶。我们的研究结果揭示了 ABA-Ca2+-CPK 信号在适应不利环境过程中调节根帽周期可塑性的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
文献相关原料
公司名称
产品信息
索莱宝
phenol-based buffer
阿拉丁
ABA
阿拉丁
ABA
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


