ZnO nanoparticles enhances cadmium tolerance by modulating N6-methyladenosine (m6A) level of stress-responsive genes NRT1 and GM35E in vegetable soybean
IF 6.1
2区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
Cadmium (Cd) is a hazardous heavy metal pollutant that poses significant risks to agricultural production and human health. Nanoparticles (NPs) can alleviate the effects of cadmium on crops by regulating the expression of stress-responsive genes, however, the mechanism of regulation is unknown. N6-methyladenosine (m6A) is a prevalent RNA modification, which determines the expression level of RNA. In this study, we performed m6A methylome analysis and gene editing to investigate the regulation of stress-responsive genes by m6A under Cd stress with ZnO nanoparticles (ZnO NPs). Firstly, we identified 16 differentially expressed genes (DEGs) with differential m6A-modification by m6A methylome seq, which included 6 stress-responsive genes. ZnO NPs treatment reduced the m6A level and stabilized the mRNAs of these stress-responsive genes. Then, we utilized the in vivo m6A modification tool, Plant m6A Editors (PMEs), specifically reduced the m6A level of NRT1 and GM35E in transgenic plants. The expression of NRT1 and GM35E was increased, and plants carrying PMEs-NRT1 and PMEs-GM35E showed stronger Cd tolerance than control. Therefore, we can conclude that NPs enhance Cd tolerance in plants by regulating the m6A methylation level of stress-responsive genes such as NRT1 and GM35E, which ultimately affects the expression of these genes. This study proposed a post-transcriptional regulatory network in vegetable soybean roots responding to Cd with ZnO NPs, potentially offering new insights into gene manipulation for controlling low Cd accumulation in crops.
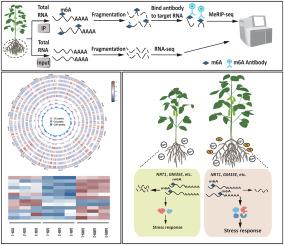
纳米氧化锌颗粒通过调节蔬菜大豆应激反应基因 NRT1 和 GM35E 的 N6-甲基腺苷(m6A)水平来增强大豆对镉的耐受性。
镉(Cd)是一种有害的重金属污染物,对农业生产和人类健康构成重大风险。纳米粒子(NPs)可以通过调节应激反应基因的表达来减轻镉对农作物的影响,但其调节机制尚不清楚。N6-甲基腺苷(m6A)是一种普遍存在的 RNA 修饰,它决定了 RNA 的表达水平。本研究通过m6A甲基化组分析和基因编辑研究了氧化锌纳米颗粒(ZnO NPs)镉胁迫下m6A对胁迫响应基因的调控。首先,我们通过m6A甲基化组测序鉴定了16个具有不同m6A修饰的差异表达基因(DEGs),其中包括6个应激反应基因。ZnO NPs处理可降低这些应激反应基因的m6A水平并稳定其mRNA。然后,我们利用体内m6A修饰工具--植物m6A编辑器(PMEs),在转基因植物中特异性地降低了NRT1和GM35E的m6A水平。结果表明,转基因植株中 NRT1 和 GM35E 的表达量增加,携带 PMEs-NRT1 和 PMEs-GM35E 的植株比对照植株表现出更强的耐镉能力。因此,我们可以得出结论:NPs 通过调节 NRT1 和 GM35E 等胁迫响应基因的 m6A 甲基化水平,最终影响这些基因的表达,从而增强植物对镉的耐受性。本研究提出了菜豆根系响应氧化锌氮氧化物镉的转录后调控网络,可能为控制作物低镉积累的基因操作提供新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.10
自引率
3.10%
发文量
410
审稿时长
33 days
期刊介绍:
Plant Physiology and Biochemistry publishes original theoretical, experimental and technical contributions in the various fields of plant physiology (biochemistry, physiology, structure, genetics, plant-microbe interactions, etc.) at diverse levels of integration (molecular, subcellular, cellular, organ, whole plant, environmental). Opinions expressed in the journal are the sole responsibility of the authors and publication does not imply the editors'' agreement.
Manuscripts describing molecular-genetic and/or gene expression data that are not integrated with biochemical analysis and/or actual measurements of plant physiological processes are not suitable for PPB. Also "Omics" studies (transcriptomics, proteomics, metabolomics, etc.) reporting descriptive analysis without an element of functional validation assays, will not be considered. Similarly, applied agronomic or phytochemical studies that generate no new, fundamental insights in plant physiological and/or biochemical processes are not suitable for publication in PPB.
Plant Physiology and Biochemistry publishes several types of articles: Reviews, Papers and Short Papers. Articles for Reviews are either invited by the editor or proposed by the authors for the editor''s prior agreement. Reviews should not exceed 40 typewritten pages and Short Papers no more than approximately 8 typewritten pages. The fundamental character of Plant Physiology and Biochemistry remains that of a journal for original results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


