Kinetic and isotherm studies on the adsorption of ionic liquids from aqueous solutions by carboxymethyl cellulose modified with sodium methacrylate sulfonate
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-12-01
DOI:10.1016/j.ijbiomac.2024.137828
引用次数: 0
Abstract
A novel carboxymethyl cellulose (CMC) graft copolymer (CMC-g-PSMAS) was successfully synthesized by grafting sodium methacrylate sulfonate (SMAS) onto CMC. The resulting CMC-g-PSMAS was used to absorb 1-allyl-3-methylimidazole chloride ([Amim]Cl) ionic liquid. The effects of different experimental factors such as monomer dosage, temperature and time on the grafting yield were systematically studied. Adsorption studies demonstrated that the adsorption equilibrium could be achieved within 60 min. The theoretical maximum adsorption capacity of CMC-g-PSMAS for [Amim]Cl reached 69.2 mg·g−1. Compared to several kinetic and isothermal models, the adsorption process of [Amim]Cl onto CMC-g-PSMAS could be well-described by the pseudo-second-order model (R2 = 0.991) and the Langmuir model (R2 = 0.999), which was a typical chemical adsorption process. Adsorption thermodynamics analyses at 25 °C revealed that the adsorption process was spontaneous (ΔG = −33.37 KJ·mol−1) and exothermic (ΔH = −56.52 KJ·mol−1). The adsorption capacity of CMC-g-PSMAS was 35.3 mg·g−1 after eight cycles, indicating its good stability and recyclability. As a consequence, CMC-g-PSMAS was efficient in the adsorption of [Amim]Cl, which could be a potential candidate for removing ionic liquids in aqueous environments.
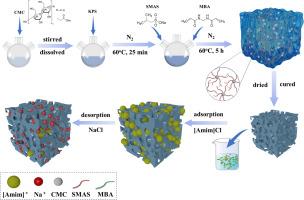
用甲基丙烯酸钠磺酸盐修饰的羧甲基纤维素对水溶液中离子液体的吸附动力学和等温线研究。
通过将甲基丙烯酸钠磺酸盐(SMAS)接枝到 CMC 上,成功合成了一种新型羧甲基纤维素(CMC)接枝共聚物(CMC-g-PSMAS)。得到的 CMC-g-PSMAS 被用来吸收 1-烯丙基-3-甲基咪唑氯化物([Amim]Cl)离子液体。系统研究了单体用量、温度和时间等不同实验因素对接枝率的影响。吸附研究表明,吸附平衡可在 60 分钟内达到。CMC-g-PSMAS 对[Amim]Cl 的理论最大吸附容量达到 69.2 mg-g-1。与几种动力学和等温模型相比,伪二阶模型(R2 = 0.991)和朗缪尔模型(R2 = 0.999)能很好地描述[Amim]Cl 在 CMC-g-PSMAS 上的吸附过程,是典型的化学吸附过程。25 °C 下的吸附热力学分析表明,吸附过程是自发的(ΔG = -33.37 KJ-mol-1)和放热的(ΔH = -56.52 KJ-mol-1)。八次循环后,CMC-g-PSMAS 的吸附容量为 35.3 mg-g-1,表明其具有良好的稳定性和可回收性。因此,CMC-g-PSMAS 能有效吸附[Aimim]Cl,可作为去除水环境中离子液体的潜在候选材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


