Diversified ring expansion of saturated cyclic amines enabled by azlactone insertion
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Saturated N-heterocycles are ubiquitous structures among natural products and biologically active compounds. Therefore, the development of synthetic methods for the construction of N-heterocycles is of great importance in the synthetic community. Altering the ring system of these motifs to analogues with different ring sizes by employing molecular editing techniques would be highly appealing in medicinal chemistry. We present herein the direct insertion of glycine derivatives as two-carbon synthons into unstrained five- or six-membered saturated cyclic amines at predictable sites, enabling the construction of synthetically challenging medium-sized azacycles through sequential Ru-catalysed C‒C bond formation, retro-aza-Michael addition and a lactamization process. Upon further derivation, we leverage this homologation platform to realize modular insertion of one- or two-carbon units into the aliphatic rings. The conversion of a single azacycle into up to five others provides a promising toolbox for diversifying existing drug candidates and increasing the prospects for clinical success. Saturated N-heterocycles are ubiquitous structures among natural products and biologically active compounds, but methods to edit the ring size of these substructures are scarce. Now the ring expansion of unactivated cyclic amines has been achieved via sequential Ru-catalysed C‒C bond formation, retro-aza-Michael addition and a lactamization process to construct synthetically challenging medium-sized azacycles.
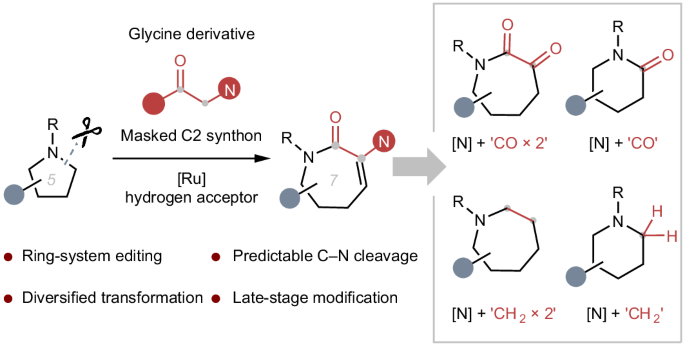
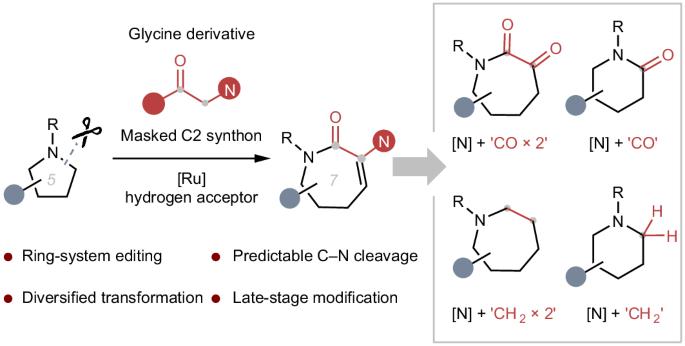
通过氮内酯插入实现饱和环胺的多样化扩环
饱和 N-杂环是天然产物和生物活性化合物中无处不在的结构。因此,开发构建 N-杂环的合成方法在合成界具有重要意义。在药物化学中,通过分子编辑技术将这些基团的环系统改变为不同环尺寸的类似物将极具吸引力。我们在本文中介绍了将甘氨酸衍生物作为两碳合成物直接插入未受约束的五元或六元饱和环胺中的可预测位点,从而通过连续的 Ru 催化 C-C 键形成、逆偶氮迈克尔加成和内酰胺化过程,构建出具有合成挑战性的中型偶氮环。在进一步衍生过程中,我们利用这一同源化平台实现了将单碳或双碳单元模块化地插入到脂肪环中。将单个氮杂环转化为多达五个其他氮杂环,为现有候选药物的多样化和提高临床成功的前景提供了一个前景广阔的工具箱。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


