Engineering of Novel Analogues That Are More Receptor-Selective and Potent than the Native Hormone, Insulin-like Peptide 5 (INSL5)
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Insulin-like peptide 5 (INSL5) targets the G protein-coupled receptor, relaxin family peptide receptor 4 (RXFP4), predominantly coexpressed in the colorectum. While INSL5 also binds to the related receptor RXFP3, it does not activate it. The INSL5/RXFP4 axis is a promising target for treating gastrointestinal disorders such as constipation. Despite its therapeutic potential, the clinical application of INSL5 has been hindered by synthesis complexities, necessitating the need for more accessible yet potent mimetics. In this study, we engineered an INSL5 analogue A13:B7-24-GG, featuring a simplified two-chain, two-disulfide scaffold with 32 amino acids, as opposed to the 45 amino acids found in native INSL5 (two-chain, three-disulfide), improving the synthesis yield by 19.5-fold. Additionally, A13:B7-24-GG demonstrates ∼4-fold higher potency (EC50 = 1.17 nM vs 4.57 nM) and ∼11 times greater selectivity than native INSL5, with significantly reduced RXFP3 binding affinity, positioning it as a promising new therapeutic candidate for the treatment of constipation.
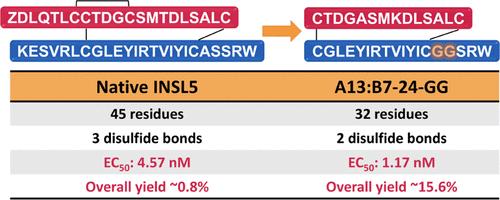
设计出比本地激素胰岛素样肽 5 (INSL5) 更具受体选择性和效力的新型类似物
胰岛素样肽 5(INSL5)靶向 G 蛋白偶联受体弛缓素家族肽受体 4(RXFP4),该受体主要在结直肠中共同表达。虽然 INSL5 也能与相关受体 RXFP3 结合,但并不能激活 RXFP3。INSL5/RXFP4 轴是治疗便秘等胃肠道疾病的一个很有前景的靶点。尽管 INSL5 具有治疗潜力,但其临床应用一直受到合成复杂性的阻碍,因此需要更容易获得的强效模拟物。在这项研究中,我们设计了一种 INSL5 类似物 A13:B7-24-GG,其特点是简化的双链、双二硫化物支架,含有 32 个氨基酸,而原生 INSL5(双链、三二硫化物)含有 45 个氨基酸,合成产量提高了 19.5 倍。此外,A13:B7-24-GG 的效力(EC50 = 1.17 nM vs 4.57 nM)比原生 INSL5 高出 4 倍,选择性比原生 INSL5 高出 11 倍,与 RXFP3 的结合亲和力显著降低,因此有望成为治疗便秘的新候选疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


