The shared genetic architecture and evolution of human language and musical rhythm
IF 21.4
1区 心理学
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
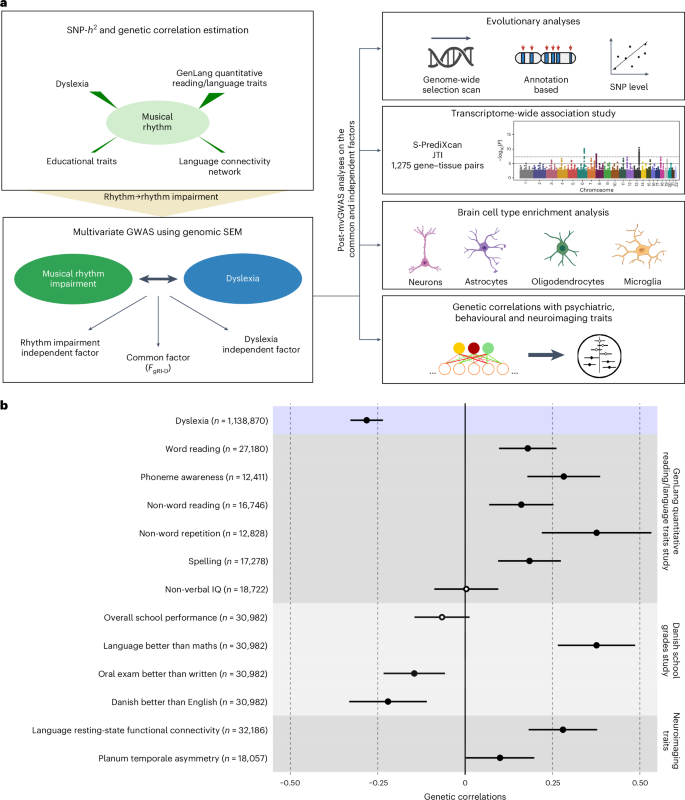
This study aimed to test theoretical predictions over biological underpinnings of previously documented phenotypic correlations between human language-related and musical rhythm traits. Here, after identifying significant genetic correlations between rhythm, dyslexia and various language-related traits, we adapted multivariate methods to capture genetic signals common to genome-wide association studies of rhythm (N = 606,825) and dyslexia (N = 1,138,870). The results revealed 16 pleiotropic loci (P < 5 × 10−8) jointly associated with rhythm impairment and dyslexia, and intricate shared genetic and neurobiological architectures. The joint genetic signal was enriched for foetal and adult brain cell-specific regulatory regions, highlighting complex cellular composition in their shared underpinnings. Local genetic correlation with a key white matter tract (the left superior longitudinal fasciculus-I) substantiated hypotheses about auditory–motor connectivity as a genetically influenced, evolutionarily relevant neural endophenotype common to rhythm and language processing. Overall, we provide empirical evidence of multiple aspects of shared biology linking language and musical rhythm, contributing novel insight into the evolutionary relationships between human musicality and linguistic communication traits. Using a battery of statistical tools, Alagöz et al. examine the genetic overlap between dyslexia and rhythm impairment and shed light on how the genome influences the neural bases of human language and musicality.

人类语言和音乐节奏的共同基因结构与进化
本研究旨在检验之前记录的人类语言相关特征与音乐节奏特征之间表型相关性的生物学基础理论预测。在确定了节奏、阅读障碍和各种语言相关性状之间的显著遗传相关性之后,我们采用多元方法捕捉节奏(N = 606 825)和阅读障碍(N = 1 138 870)全基因组关联研究中常见的遗传信号。研究结果显示,16 个多效应位点(P < 5 × 10-8)与节奏障碍和阅读障碍共同相关,并具有错综复杂的共同遗传学和神经生物学结构。联合遗传信号富集于胎儿和成人脑细胞特异性调控区域,凸显了它们共同基础中复杂的细胞组成。与一个关键白质束(左上纵筋束-I)的局部遗传相关性证实了关于听觉-运动连通性的假设,这是一种受遗传影响的、与节奏和语言处理共同进化相关的神经内表型。总之,我们提供了经验证据,证明语言和音乐节奏之间存在多方面的共同生物学联系,为人类音乐性和语言交流特征之间的进化关系提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Human Behaviour
Psychology-Social Psychology
CiteScore
36.80
自引率
1.00%
发文量
227
期刊介绍:
Nature Human Behaviour is a journal that focuses on publishing research of outstanding significance into any aspect of human behavior.The research can cover various areas such as psychological, biological, and social bases of human behavior.It also includes the study of origins, development, and disorders related to human behavior.The primary aim of the journal is to increase the visibility of research in the field and enhance its societal reach and impact.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


