An enantioselective HAT for diols
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
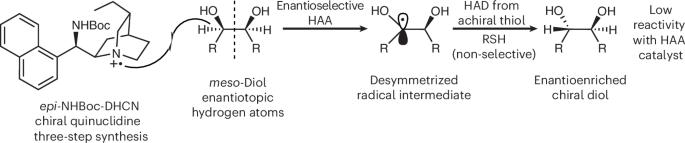
二元醇的对映选择性 HAT
研究小组探索了催化剂如何在光催化剂1,2,3,5-四(咔唑-9-基)-4,6-二氰基苯(4CzIPN)和蓝光照射下,在顺式-1,2-环己二醇上生成自由基中间体,该中间体可被作为氢原子供体的1-十二硫醇捕获,生成反式-1,2-环己二醇。该反应对不同大小、具有融合环或带有丙酮、酯或苄基的环状二元醇具有耐受性;它还成功地应用于无环状二元醇,如中-己烷-3,4-二醇、中-1,3-二醇和戊烷-2,4-二醇。研究小组还证明,催化剂可以控制丙烯酰胺或乙烯基砜与二元醇基质上生成的自由基进行 Giese 加成时的对映体选择。机理研究证实,光激发的 4CzIPN 会被催化剂淬灭,产生的自由基阳离子会使二元醇发生非对称反应,形成自由基中间体,从而使反应处于动力学控制之下。该中间体经过硫醇的非选择性氢原子传递,生成手性二元醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


