Radical-mediated regiodivergent C(sp3)–H functionalization of N-substituted indolines via enzymatic carbene transfer
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
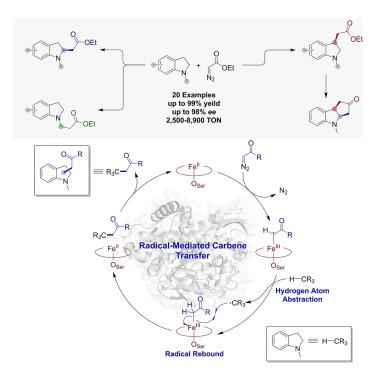
Indolines are ubiquitous structural motifs occurring in pharmaceuticals and natural products. Here, we report a strategy for regio- and stereoselective C(sp3)–H functionalization of N-substituted indolines via carbene transfer chemistry mediated by engineered CYP119-based catalysts. These systems offer high enantioselectivity and high catalytic efficiency, as well as regiodivergent selectivity, furnishing an efficient and convenient route for diversification of these important scaffolds via direct C(sp3)–H functionalization. Selective functionalization of exocyclic C(sp3)–H bond in N-methyl indolines was also achieved, and a biocatalytic cascade combining enzyme-mediated α- and β-C(sp3)–H functionalization yielded a polycyclic indoline-containing motif found in drugs. Mechanistic and computational studies support a radical-mediated C–H functionalization pathway and provide insights into protein-mediated regiodivergent selectivity. Altogether, this work offers a direct and tunable strategy to access functionalized indolines as key building blocks for medicinal chemistry and natural product synthesis and provides first insights into the mechanism of P450-catalyzed C(sp3)–H carbene insertion.
通过酶促碳烯转移对 N-取代吲哚啉进行自由基介导的 C(sp3)-H 功能化
吲哚类化合物是药物和天然产物中无处不在的结构基团。在此,我们报告了一种在基于 CYP119 的工程催化剂介导下,通过碳烯转移化学对 N-取代吲哚啉进行区域和立体选择性 C(sp3)-H官能化的策略。这些系统具有高对映选择性、高催化效率和区域选择性,为通过直接 C(sp3)-H 功能化实现这些重要支架的多样化提供了一条高效便捷的途径。此外,还实现了对 N-甲基吲哚啉中的外环 C(sp3)-H 键的选择性官能化,并通过结合酶介导的 α- 和 β-C(sp3)-H 官能化的生物催化级联产生了药物中的多环吲哚啉基团。机理和计算研究支持自由基介导的 C-H 功能化途径,并为蛋白质介导的区域选择性提供了见解。总之,这项工作为获得功能化吲哚啉作为药物化学和天然产物合成的关键构件提供了一种直接和可调整的策略,并首次揭示了 P450 催化 C(sp3)-H 碳烯插入的机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


