Enhanced electrochemical reduction of CO2 to CO by ZnO nanorods enriched with oxygen vacancies
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The efficiency of converting CO2 to other valuable chemicals via the electrochemical reduction pathway depends on the electrocatalyst. In this work, an approach to prepare the ZnO catalysts used for the CO2 electrocatalytic reduction was proposed, aiming at regulating the oxygen vacancy concentration in ZnO nanorods by changing the heat treatment temperature. The results show that the faradaic efficiency of CO2 reduction to CO is significantly improved. An unprecedented faradaic efficiency of 98.3% and a current density of 786.56 mA cm−2 were achieved using the ZnO catalyst heat treated at 500°C. It is revealed that the oxygen vacancy concentration, combined with density functional theory, can improve the performance of the ZnO electrocatalytic reduction of carbon dioxide (CO2RR) by accelerating the activation of CO2 molecules and reducing the energy barrier of CO formation. This work is helpful for the development of robust and efficient ZnO catalysts and their application in the CO2RR.
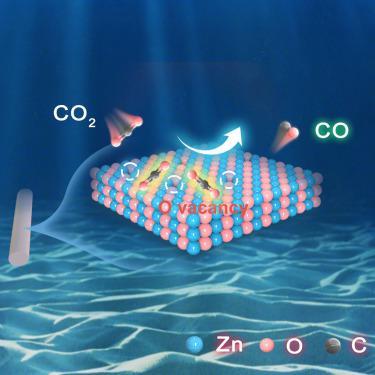
富含氧空位的氧化锌纳米棒增强了将 CO2 还原成 CO 的电化学过程
通过电化学还原途径将二氧化碳转化为其他有价值化学品的效率取决于电催化剂。在这项工作中,提出了一种制备用于二氧化碳电催化还原的氧化锌催化剂的方法,旨在通过改变热处理温度来调节氧化锌纳米棒中的氧空位浓度。结果表明,二氧化碳还原成一氧化碳的远红外效率显著提高。在 500°C 下热处理的氧化锌催化剂达到了前所未有的 98.3% 的法拉第效率和 786.56 mA cm-2 的电流密度。研究表明,氧空位浓度与密度泛函理论相结合,可以通过加速二氧化碳分子的活化和降低二氧化碳形成的能量障碍来改善氧化锌电催化还原二氧化碳(CO2RR)的性能。这项工作有助于开发稳健高效的氧化锌催化剂并将其应用于 CO2RR。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


