The coordination chemistry and anticancer activity of organo-ruthenium(II), -iridium(III) and -rhodium(III) complexes with sulfonyl-substituted thiourea ligands
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
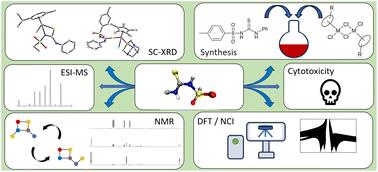
Some half-sandwich compounds with a variety of ligands and metal centres have shown promising anticancer activity. Herein we report a series of reactions between the sulfonylthiourea ligands p-TolSO2NHC(S)NHPh, EtSO2NHC(S)NHPh and CH3SO2NHC(S)NHPh and [(η6-p-cymene)RuCl2]2, [(η6-arene)RuCl2(PR3)] (arene = benzene or p-cymene), [Cp*MCl2(PR3)] or [Cp*RhCl2]2 (M = Ir(III), Rh(III)), Cp* = η5-pentamethylcyclopentadienyl, PR3 = triphenylphosphine (PPh3), tris(2-cyanoethyl)phosphine (tcep) and 1,3,5-triaza-7-phosphaadamantane (pta) and their corresponding piano stool complexes. Single crystal X-ray diffraction structure determinations indicated that the resulting linkage isomer of the complex, i.e., proximal (coordination via S,N(sulfonated) placing the sulfonyl group near the coordination sphere) or distal (coordination via S,N(non-sulfonated), placing the sulfonyl group away from the coordination sphere), is directly related to the steric bulk around the metal centre. Proximal to distal isomerisation of the complex [(η6-p-cymene)Ru{p-TolSO2NC(S)N(PPh3)}] (1aL1) was observed by 1H and 31P{1H} NMR spectroscopy. DFT calculations suggested this to be the result of the conversion from the initially formed kinetically favourable to the thermodynamically favourable isomer. Computational investigation of non-covalent interactions using the reduced density gradient also revealed a chalcogen bond present between the thiourea sulfur and sulfonyl oxygen atoms of complex 1aLa. The in vitro antiproliferative activity of several complexes was determined against human cancer cells, which revealed a correlation between potency and lipophilic properties of the ancillary ligands for a series of Ru(II) p-cymene complexes.
磺酰基取代的硫脲配体的有机钌(II)、铱(III)和铑(III)配合物的配位化学和抗癌活性
一些含有多种配体和金属中心的半三明治化合物显示出了良好的抗癌活性。在此,我们报告了磺酰硫脲配体 p-TolSO2NHC(S)NHPh、EtSO2NHC(S)NHPh 和 CH3SO2NHC(S)NHPh 与[(η6-p-cymene)RuCl2]2、[(η6-arene)RuCl2(PR3)](arene = 苯或对-cymene)之间的一系列反应、[Cp* = η5-五甲基环戊二烯,PR3 = 三苯基膦 (PPh3)、三(2-氰乙基)膦 (tcep) 和 1,3,5-三氮杂-7-磷杂金刚烷 (pta) 以及它们相应的钢琴凳配合物。单晶 X 射线衍射结构测定结果表明,复合物的连接异构体,即近端(通过 S,N(磺化)配位,将磺酰基置于配位球附近)或远端(通过 S,N(非磺化)配位,将磺酰基置于远离配位球的位置),与金属中心周围的立体空间直接相关。通过 1H 和 31P{1H} NMR 光谱观察到复合物 [(η6-对伞花烃)Ru{p-TolSO2NC(S)N(PPh3)}] (1aL1) 从近端到远端的异构化。NMR 光谱观测到。DFT 计算表明,这是最初形成的动力学上有利的异构体向热力学上有利的异构体转化的结果。利用还原密度梯度对非共价相互作用进行的计算研究还发现,在复合物 1aLa 的硫脲硫原子和磺酰氧原子之间存在一个缩醛键。研究还测定了几种复合物对人类癌细胞的体外抗增殖活性,结果表明一系列 Ru(II) 对伞花烃复合物的有效性与辅助配体的亲脂性之间存在相关性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


