Tailoring the electronic conductivity of coating layer on the composite separator for Li metal anode
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Protection of lithium (Li) metal anodes is essential for the high performance of lithium metal batteries. Although the SnO2 coating layer on the composite separator can provide protection for the Li metal anode, the effect diminishes over time as Li is plated on the coating surface, which can be attributed to the increased electronic conductivity of the reduced SnO2 artificial solid electrolyte interfacial layer. Here, poly(vinylidene fluoride) (PVDF) is blended with SnO2 to form an insulated SnO2/PVDF hybrid coating layer. The SnO2/PVDF hybrid coating layer significantly mitigates initial capacity loss and enhances the Coulombic efficiency of Cu||Li batteries. Adjustments to electronic conductivity of coating layer result in the deposition of large grain size Li beneath the hybrid coating layer. The SnO2/PVDF (=7/3) (S7P3) layer can stabilize the freshly deposited Li and improve the cycling performance of the Cu@Li/S7P3 electrode. The S7P3@PE composite separator can significantly increase the cycle performance of LiFePO4 (LFP)||Li batteries under low-temperature conditions. Furthermore, the S7P3 layer can provide substantial advantages to LFP batteries with limited Li capacity, as well as to lithium-free anodes. Our approach to regulating Li deposition behavior by tailoring the electronic conductivity of the coating layer facilitates the long-term stability of the Li metal anode.
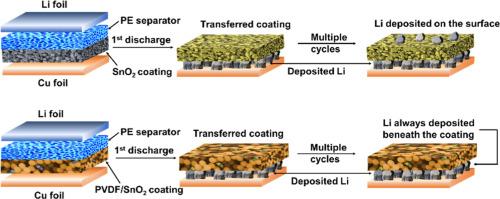
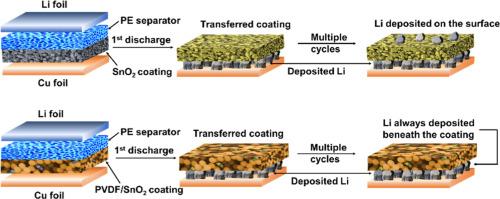
调整锂金属阳极复合分离器涂层的电子导电性
保护锂(Li)金属阳极对锂金属电池的高性能至关重要。虽然复合隔膜上的二氧化锡涂层可以为锂金属阳极提供保护,但随着时间的推移,涂层表面镀上锂的效果会逐渐减弱,这可能是由于还原的二氧化锡人工固态电解质界面层的电子导电性增加所致。在这里,聚偏二氟乙烯(PVDF)与二氧化锡混合形成了绝缘的二氧化锡/PVDF 混合镀膜层。SnO2/PVDF混合镀膜层可显著减少初始容量损失,并提高铜||锂电池的库仑效率。调整镀膜层的电子导电性可在混合镀膜层下沉积大晶粒锂。SnO2/PVDF(=7/3)(S7P3)层可以稳定新沉积的锂,提高 Cu@Li/S7P3 电极的循环性能。S7P3@PE 复合隔膜可显著提高磷酸铁锂(LFP)||锂电池在低温条件下的循环性能。此外,S7P3 层还能为锂容量有限的 LFP 电池以及无锂阳极提供实质性优势。我们通过调整镀膜层的电子导电性来调节锂沉积行为的方法有利于锂金属阳极的长期稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


